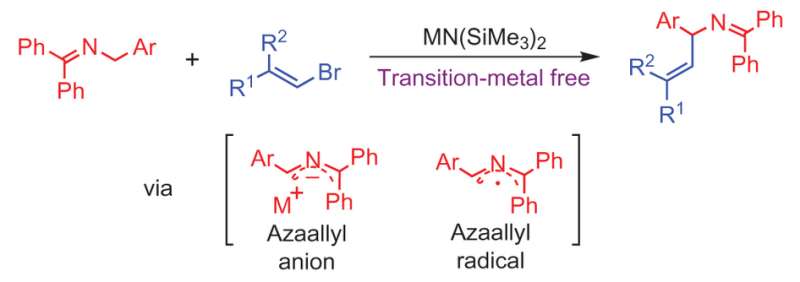
Transition-metal-free vinylation of azaallyl anions. Credit: (c) Nature Chemistry (2017). DOI: 10.1038/nchem.2760
(Phys.org)—Certain functional groups tend to show up often in natural products and biologically relevant molecules. Among those functional groups are allylic amines. The typical protocols for the synthesis of allylic amines involve a carbon-carbon coupling reaction that requires a transition metal catalyst. However, transition metal catalysts tend to be expensive, particularly if a reaction is done at a commercial scale. Furthermore, companies are interested in green alternatives to transition metal catalysis.
Researchers at the University of Pennsylvania have developed a mechanism for making allylic amines without the use of transition metal catalysts. Their work is the first reported instance of transition-metal-free C(sp3)-C(sp2) coupling of vinyl bromide electrophiles with azaallyl anions and azaallyl radicals. Their work appears in Nature Chemistry.
"This work opens up several new avenues that could affect many types of transformations," says Professor Marisa C. Kozlowski one of the principle authors of the study. "The formation of the radical species by a carbanion donating an electron shows a non-metallic entry to these important reactive species. In addition, this chemistry adds a distinct mechanism in cross-coupling allowing certain architectures to be generated more efficiently."
Building on their prior work for making allylic amines via a 1,1-diphenyl-3-arylallyl-2-azaallyl anion , Li et al. discovered that when they reacted an ketimine with a vinyl bromide to form their azaallyl anion, the subsequent vinylation reaction occurred without the need for a palladium catalyst. The sp2 carbon on the vinyl halide added to the sp3 carbon on the aryl azaallyl anion (See figure). This type of carbon-carbon coupling reaction typically needs a transition metal catalyst.
By using a base that is sterically hindered, [MN(SiMe3)2 where M = Li, Na], the reaction does not deprotonate the product and results in the E-vinylation product in good yield. This mechanism is regioselective, reacting with the imine carbon, and chemoselective for the formation of the allylic amine over the competing reaction that forms a terminal alkyne from the vinyl bromide.
Once Li et al. optimized their reaction conditions, they tested the scope of their new mechanism. In general, their mechanism is successful with a range of functional groups on the N-benzyl amine and on the vinyl bromide. The authors report that they did have to adjust some of the reaction conditions depending on the functional groups on the N-benzyl amine, but, overall, the reaction worked for electron withdrawing groups, such as 4-halides and 3,5-di-CF3. Additionally, this reaction worked for electron donating groups, such as 4-methyl and with heterocyclic compounds, such as pyridyl ketimines and thiophenyl ketimines.
The authors then looked at the versatility of the vinyl bromide. Their reaction tolerated a broad range of vinyl bromide products including several aryl vinyl bromides and aliphatic vinyl bromides. Importantly, the authors did not detect isomerization and cyclization with any of the vinyl bromides tested.
Computational and experimental studies were conducted to better understand the mechanism in hopes of testing other types of functional groups in the future. Li et al. eventually determined a mechanism that involved an unusual radical intermediate formed from azaallyl anion electron transfer after deprotonation of the ketimine. Electron paramagnetic resonance studies confirmed that there is a radical species involved, but further studies are needed to understand the nature of this species and whether the radical(s) is directly involved in the vinylation reaction.
The authors believe the radical is likely an azaallyl radical and that both the azaallyl anion and azaallyl radical are intermediates in a mechanism that is dependent upon the substrate on the vinyl bromide. However, additional studies would need to be conducted to confirm this.
This research opens the door to the production of natural products without the use of a transition metal catalyst for a reaction that typically requires transition metals. Further research will look at the versatility of this reaction and its subsequent application.
More information: Minyan Li et al. Transition-metal-free chemo- and regioselective vinylation of azaallyls, Nature Chemistry (2017). DOI: 10.1038/nchem.2760
Abstract
Direct C(sp3)–C(sp2) bond formation under transition-metal-free conditions offers an atom-economical, inexpensive and environmentally benign alternative to traditional transition-metal-catalysed cross-coupling reactions. A new chemo- and regioselective coupling protocol between 3-aryl-substituted-1,1-diphenyl-2-azaallyl derivatives and vinyl bromides has been developed. This is the first transition-metal-free cross-coupling of azaallyls with vinyl bromide electrophiles and delivers allylic amines in excellent yields (up to 99%). This relatively simple and mild protocol offers a direct and practical strategy for the synthesis of high-value allylic amine building blocks that does not require the use of transition metals, special initiators or photoredox catalysts. Radical clock experiments, electron paramagnetic resonance studies and density functional theory calculations point to an unprecedented substrate-dependent coupling mechanism. Furthermore, an electron paramagnetic resonance signal was observed when the N-benzyl benzophenone ketimine was subjected to silylamide base, supporting the formation of radical species upon deprotonation. The unique mechanisms outlined herein could pave the way for new approaches to transition-metal-free C–C bond formations.
Journal information: Nature Chemistry
© 2017 Phys.org