June 1, 2016 report
Safe and generalizable catalyst for carbonyl-olefin metathesis reaction
(Phys.org)—Olefin metathesis reactions where two allyls switch substituent groups, has proved to be a useful carbon-carbon bond forming reaction for drug discovery and other industrial processes. The carbonyl-olefin metathesis reaction was discovered around the same time as the olefin metathesis reaction, but has had limited applicability due to harsh reaction conditions and stringent reactant requirements. Until now, there have been no examples of catalysts for this reaction that are generalizable.
In a report in Nature, Jacob R. Ludwig, Paul M. Zimmerman, Joseph B. Gianino & Corinna S. Schindler of the University of Michigan have successfully found a Lewis acid catalyst that successfully exacts a carbonyl-olefin ring-closing metathesis reaction for the direct synthesis of functionalized carbocycles. Furthermore, the catalyst uses iron, which is abundant and environmentally safe, and works under mild conditions for a variety of functional groups and at gram-level scales.
The olefin reaction mechanism involves a metal-catalyzed [2+2] cycloaddition of two allyls to form a four-member cyclic intermediate in which the metal is one of the members of the four-member ring. This is followed by a [2+2] cycloreversion resulting in products that have exchanged substituents. Carbonyl olefin metathesis reactions operate similarly. There are three carbonyl olefin metathesis reactions have been observed, but each has its limitations. One involves a two-step photochemical cycloaddition followed by a thermolysis cycloreversion. Another involves a stoichiometric amount of transition-metal catalyst. Another reaction involves an organocatalyst that requires a cyclopropene reactant. All of these are limited in scope and, in the case of the photochemical reaction, require prohibitively harsh conditions. The reaction involving a stoichiometric amount of transition-metal catalyst is the most versatile, but it produces a metal-oxo complex that consumes the catalyst.
Ludwing, et al. wanted to look for ways to form an in situ oxetane intermediate, a four-member cyclic intermediate with the oxygen as one of the atoms instead of a metal. This would avoid the formation of products that consume the catalyst. In general, they needed a catalyst that promotes [2+2] cycloaddition and [2+2] cyclorevrsion, does not promote side reactions such as polymerization or reforming reactants, and is chemoselective. They decided to investigate metal-derived Lewis acids.
To test various Lewis acid catalysts, they reacted a β-ketoester with a pendant isoprenyl group with MClx Lewis acids. They tested AlCl3, TiCl4, SnCl4, FeCl2, ZnCl2, SnCl2, and FeCl3. They found that FeCl3 formed the desired products and that optimal reaction conditions were 5 mol% FeCl3 in dicholorethane at room temperature for one-to-twelve hours.
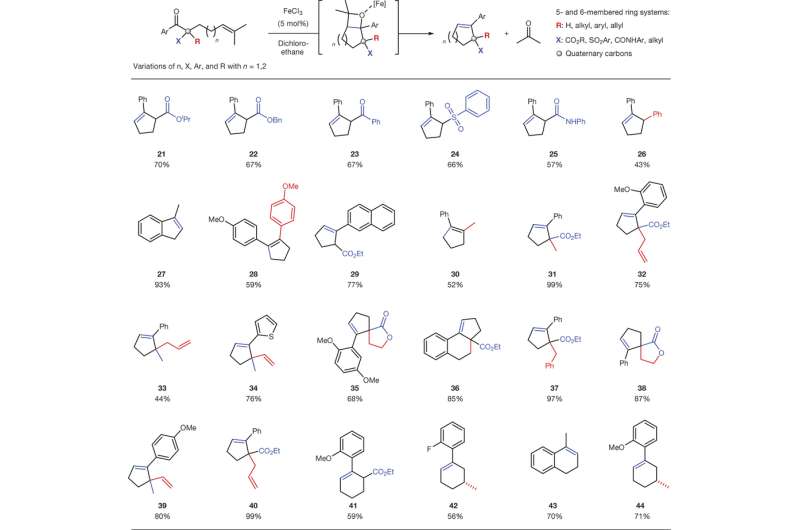
Once a catalyst was determined and reaction conditions were optimized, the next step was to test the generalizability of the reaction. Ludwig, et al. looked at various arene groups. Their reaction conditions worked with electron withdrawing and donating groups producing products in good yield. They then changed the β-substitution on the β-ketoesters and found the reaction worked with a variety of substituents. They also looked at the effects of incorporating a heteroatom, changing structural motifs, and changing the pendant isoprenyl group. Overall, the FeCl3 catalyst showed remarkable versatility in the variety of reactions that were successfully completed.
These reactions provided insight into the carbonyl-olefin reaction mechanism. The reaction either proceeds via a carbocation intermediate or in a concerted fashion that involves metal coordination to FeCl3. Subsequent studies using a mechanistic probe to trap the product that would be formed from a carbocation intermediate showed no results, thus demonstrating that a concerted and metal coordinated mechanism is likely. Computational studies indicate that the reaction mechanism is likely a concerted carbon-oxygen bond breakage and carbon-carbon bond formation mechanism that occurs asynchronously.
Carbonyl-olefin metathesis reactions had been limited in scope, but this study demonstrates a catalyst and optimal reaction conditions that are generalizable for a variety of reactants opening the door for the synthesis of complex compounds. The reaction is practically useful as iron is an earth-abundant metal and is environmentally safe, and the reactions are scalable.
More information: Jacob R. Ludwig et al. Iron(III)-catalysed carbonyl–olefin metathesis, Nature (2016). DOI: 10.1038/nature17432
Abstract
The olefin metathesis reaction of two unsaturated substrates is one of the most powerful carbon–carbon-bond-forming reactions in organic chemistry. Specifically, the catalytic olefin metathesis reaction has led to profound developments in the synthesis of molecules relevant to the petroleum, materials, agricultural and pharmaceutical industries1. These reactions are characterized by their use of discrete metal alkylidene catalysts that operate via a well-established mechanism2. While the corresponding carbonyl–olefin metathesis reaction can also be used to construct carbon–carbon bonds, currently available methods are scarce and severely hampered by either harsh reaction conditions or the required use of stoichiometric transition metals as reagents. To date, no general protocol for catalytic carbonyl–olefin metathesis has been reported. Here we demonstrate a catalytic carbonyl–olefin ring-closing metathesis reaction that uses iron, an Earth-abundant and environmentally benign transition metal, as a catalyst. This transformation accommodates a variety of substrates and is distinguished by its operational simplicity, mild reaction conditions, high functional-group tolerance, and amenability to gram-scale synthesis. We anticipate that these characteristics, coupled with the efficiency of this reaction, will allow for further advances in areas that have historically been enhanced by olefin metathesis.
Journal information: Nature
© 2016 Phys.org