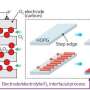
This science illustration demonstrates the collapsing of the balloon-shaped-reaction product during the charging of the lithium-oxygen battery. Credit: Environmental Molecular Sciences Laboratory
For the lithium-oxygen battery system, it is well recognized the charging and discharging reaction produces peculiar reaction product shapes that resemble doughnuts and balloons. Yet, how these shapes form has remained a mystery. A new study of a functioning nano-lithium-oxygen battery at atomic scale in an oxygen atmosphere provides clues for solving this mystery.
The discovery of the lithium-oxygen reaction pathway sets the foundation for quantitative modeling of electrochemical processes in the lithium-oxygen system, providing insight into how best to design lithium-oxygen batteries with high capacity and longer cycle life.
The lithium-oxygen battery system has been perceived as an enabling technology for the electromotive industry. However, progress in research and development of a lithium-oxygen battery has been severely hampered by two unanswered questions. First, what is the electrochemical reaction route when discharging and charging the battery? Second, what is the relationship between the complicated shapes of the reaction product and the reaction path? Answers to these two questions are fundamental, yet essential for development of the lithium-oxygen batteries.
To address this knowledge gap, a team of researchers from Pacific Northwest National Laboratory; Tianjin Polytechnic University of China; and EMSL, the Environmental Molecular Sciences Laboratory, used advanced in-situ imaging techniques—the environmental transmission electron microscope—at EMSL, a Department of Energy Office of Science user facility, to observe a nano-lithium-oxygen battery during charging and discharging. They found oxygen reacts with lithium on carbon nanotubes to form a metastable lithium oxide.
This oxide transforms into a more stable lithium oxide and releases oxygen gas that expands (inflates) particles into a hollow structure, producing doughnut and balloon shapes. This observation more generally demonstrates that the way the released oxygen is accommodated governs the formation of the complicated morphology of the reaction product in a lithium-oxygen battery. The results of this work not only answer the two questions outlined above, but also provide insight into ion and electron transport coupled with mass flow for the lithium-oxygen battery.
More information: Langli Luo et al. Revealing the reaction mechanisms of Li–O2 batteries using environmental transmission electron microscopy, Nature Nanotechnology (2017). DOI: 10.1038/NNANO.2017.27
Journal information: Nature Nanotechnology
Provided by Environmental Molecular Sciences Laboratory