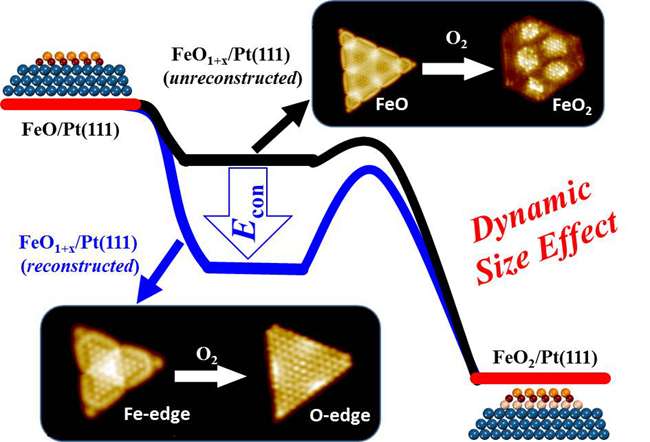
A schematic illustration of the dynamic size effect in enhancing the oxidation resistance of active FeO NSs. Credit: YANG Fan
The research group led by Prof. BAO Xinhe from Dalian Institute of Chemical Physics, Chinese Academy of Sciences, has discovered that oxide nanostructures (NSs) with a diameter below 3 nm could exhibit superior oxidation resistance to larger NSs. By investigating the oxidation mechanism at the atomic level, the team has proposed, for the first time, a "dynamic size effect" that determines the stability of supported nanoparticles.
These findings were published in the latest issue of Nature Communications titled "Enhanced oxidation resistance of active nanostructures via dynamic size effect." This study not only lends atomic understanding of the dynamic remodeling mechanism of nanocatalysts in oxygen, but also provides a new interface control for the development of an anti-corrosion and anti-oxidation nano-protective coating.
A major challenge limiting the practical applications of nanomaterials is that the activities of NSs increase with reduced size, often losing stability in the chemical environment. Under oxidative conditions, NSs with smaller sizes and higher defect densities are commonly expected to oxidize more easily, since high-concentration defects can facilitate oxidation by enhancing the reactivity with O2 and providing a fast channel for oxygen incorporation.
Previously, several nanocrystalline materials were also reported to exhibit improved oxidation resistance with respect to bulk materials and have been applied as anti-corrosion coatings. The lack of general consensus on the oxidation resistance of oxide NSs has been attributed to the limited understanding of the underlying mechanism of oxidation, particularly, the oxidation kinetics of NSs with diameters below 5 nm, which have rarely been studied.
BAO's team and Prof. YANG Fan thus constructed FeO NSs with different sizes on Pt (111) and studied their oxidation kinetics using high-resolution scanning tunneling microscopy (STM) and density-functional theory (DFT) calculations. Reducing the size of active FeO NSs was found to increase their oxidation resistance drastically, and a maximum oxidation resistance was found for FeO NSs with dimensions below 3.2 nm. The team found the enhanced oxidation resistance originates from the size-dependent structural dynamics of FeO NSs in O2.
Specifically, the study shows that FeO NSs with a size below 3.2 nm could undergo a facile and complete reconstruction, when O2 dissociates at the coordinatively unsaturated ferrous centers at the edges of FeO NSs. Accompanying the reconstruction, the dissociated oxygen atoms are stabilized at the edges of FeO NSs and could not penetrate into the interface FeO and Pt, thereby inhibiting the further oxidation of FeO NSs. FeO NSs with dimensions above 3.2 nm oxidize more easily because of their inability to complete the reconstruction, accompanied by the formation of surface dislocations.
In other words, small FeO NSs are more susceptible to dynamic changes in the reaction, to achieve a relatively stable structure. The authors term this as the "dynamic size effect" and found it to govern the chemical properties of active NSs. To demonstrate the generality of dynamic size effect, the researchers also studied CoO NSs supported on Pt (111) or Au (111), and found similar oxidation-resistant behavior for NSs below 3 nm.
More information: Yun Liu et al, Enhanced oxidation resistance of active nanostructures via dynamic size effect, Nature Communications (2017). DOI: 10.1038/ncomms14459
Journal information: Nature Communications
Provided by Chinese Academy of Sciences