Credit: American Chemical Society
In pictures, the Arctic appears pristine and timeless with its barren lands and icy landscape. In reality, the area is rapidly changing. Scientists are working to understand the chemistry behind these changes to better predict what could happen to the region in the future. One team reports in ACS' Journal of Physical Chemistry A that sea salt could play a larger role in the formation of local atmospheric pollutants than previously thought.
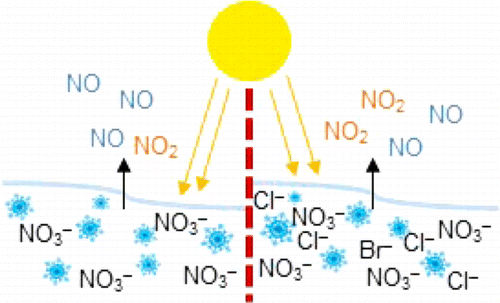
The Arctic's wintertime ice hit a record low this year, and its air is warming, according to NASA. Previous research has shown that pollutants, including gaseous nitrogen oxides and ozone, have at times been recorded at levels similar to those one would see in more populated areas. Nitrogen oxides are air pollutants that, in sunlight, lead to the formation of ozone, the main component in smog normally associated with cities. The gases can be processed in the atmosphere and be deposited on Earth as nitrates, which can get trapped in snow. In sunlight, snow can act as a reactor in which nitrates may be transformed back to nitrogen oxide gases. In the Arctic, sea ice and snow contain salt and other impurities that can possibly alter the efficiency of this process. James Donaldson, Karen Morenz and colleagues took a closer look at how salt and nitrate content in snow could affect the levels of nitrogen oxides in the air during sunny conditions.
The researchers tested lab-made snow containing nitrate alone or nitrate and salt. They found that under simulated sunlight, about 40 to 90 percent more nitrogen dioxide (NO2) was reformed from the snow with low levels of salt at environmentally relevant concentrations than snow with no salt. Researchers observed the greatest effect when they used realistic sea salt in the experiment. The results suggest that sea ice and salty snow, which previously have not been considered as factors in the balance of ozone-forming chemicals in the atmosphere, should be a part of future models.
More information: Karen J. Morenz et al. Nitrate Photolysis in Salty Snow, The Journal of Physical Chemistry A (2016). DOI: 10.1021/acs.jpca.6b06685
Abstract
Nitrate photolysis from snow can have a significant impact on the oxidative capacity of the local atmosphere, but the factors affecting the release of gas-phase products are not well understood. Here, we report a systematic study of the amounts of NO, NO2, and total nitrogen oxides (NOy) emitted from illuminated snow samples as a function of both nitrate and total salt (NaCl and Instant Ocean) concentration. The results provide experimental evidence that the release of nitrogen oxides to the gas phase is directly related to the expected nitrate concentration in the brine at the surface of the snow crystals. With no added salts, steady-state release of gas-phase products increases to a plateau value with increasing prefreezing nitrate concentration; with the addition of salts, the steady-state gas-phase nitrogen oxides generally decrease with increasing prefreezing NaCl or Instant Ocean concentration. In addition, for these frozen mixed nitrate (25 mM)–salt (0–500 mM) solutions, there is an increase in gas-phase NO2 seen at low added salt amounts, with NO2 production enhanced by up to 42% at low prefreezing [NaCl] (≤25 mM) and by up to 89% at prefreezing Instant Ocean concentrations lower than 200 mM [Cl–]. This enhancement may be important to the atmospheric oxidative capacity in polar regions.
Journal information: Journal of Physical Chemistry A
Provided by American Chemical Society