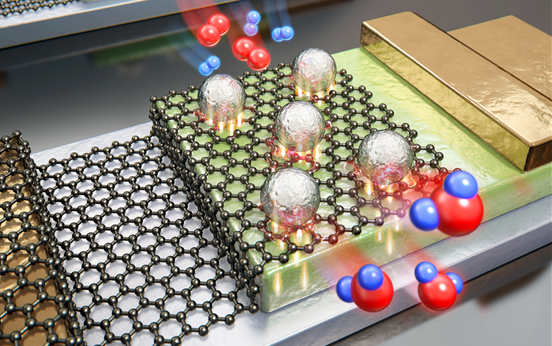
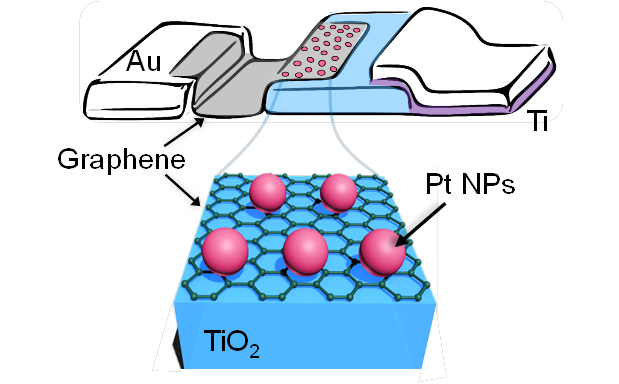
Schottky junction between a single layer of graphene and an n-type TiO2 layer lowered potential barrier existing at the Pt NPs/graphene interface, allowing the detection of hot electron flows produced during H2O formation. Credit: IBS
From converting vehicle exhaust fumes into less harmful gases to refining petroleum, most commercial chemical applications require nanocatalysts since they can reduce the required time and costs by controlling the rate of chemical reactions. The catalytic activity and selectivity largely depends on their physical properties (size, shape, and composition) as well as the electronic characteristics; the dynamics of hot (high energy) electrons on the surface and interface of catalysts. Though the catalyst industry is constantly growing, it's challenging to permit electric currents to nanocatalysts in order to detect hot electrons and measure the catalytic efficiency.
In a new study, the Institute for Basic Science (IBS) team working under the Center's group leader, Professor PARK Jeong Young, created a catalytic nanodiode composed of a single layer of graphene and titanium film (TiO2) that enabled the detection of hot electrons on platinum nanoparticles (Pt NPs). This breakthrough research developed a catalytic nanodiode that allowed the team to observe in real time the flow of hot electrons generated by chemical reactions. Since hot electrons are created when excess energy from the surface of a chemical reaction is permitted to dissipate in femtosecond, they are deemed as an indicator for the catalystic activity. However, the quick thermalization of hot electrons makes the direct detection of hot electrons quite difficult for clarifying the electronic effect on catalytic activity on metal nanoparticles. In this study, researchers extracted 'hot carriers' from a metal catalyst using a graphene-semiconductor junction.
A new approach
The scientific team experiments differed to previous attempts where gold was used which proved to be inefficient, unstable and expensive. The team from the Center for Nanomaterials and Chemical Reactions experimented on a single layer of graphene, grown on a copper film before being transported to TiO2 where Pt NPs were later deposited. Graphene, the 2D wonder material, was used because of its unique electronic and chemical properties. When integrated with metal NPs, tremendous improvements in the conductivity performance between the supporting material and the platinum NPs were observed by the team. The catalytic activity and amount of hot electrons were measured; the results showed that the catalytic activity and the generation of hot electrons are well-matched and the reaction mechanism can be studied with hot electrons dynamic. "Graphene-based nanostructures, such as ours are promising detectors for the study of hot electron dynamics on metal NPs during the course of catalytic reactions" confirmed the team's paper.
A photograph of graphene-based catalytic nanodiodes. Credit: IBS
The team's work, according to their paper, highlights the lowered contact resistance at the Pt NPs/ graphene interface is the main characteristic leading to efficient hot electron detection on the nanocatalysts in the graphene- based catalytic nanodiode. By utilizing a single layer of graphene for electrical connection of the Pt NPs it allowed for easier observation of hot electrons because of both the atomically thin nature of graphene and the reduced height of the potential barrier existing at the Pt NPs/ graphene interface. The research conducted at IBS can, potentially, help design catalytic and energy materials with improved performances and lower costs. First author and Ph.D. student Hyosun LEE stated: "Even though there is still the potential for improving the quality of the graphene layer itself and its contact with the TiO2, the approach presented here offers a new way to study the roles of graphene during heterogeneous catalysis."
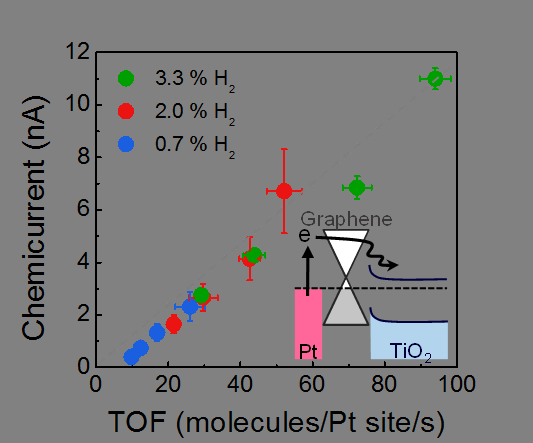
A chemicurrent as a function of TOF for H2 oxidation, measured at different concentrations of H2. Credit: IBS
More information: Hyosun Lee et al. Graphene–Semiconductor Catalytic Nanodiodes for Quantitative Detection of Hot Electrons Induced by a Chemical Reaction, Nano Letters (2016). DOI: 10.1021/acs.nanolett.5b04506
Journal information: Nano Letters
Provided by Institute for Basic Science