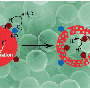
Ferrite boosting photocatalytic hydrogen evolution
Photocatalytic hydrogen generation via water splitting has become a hot spot in the field of energy and materials. The goal of this technique is to construct cheap and efficient photocatalytic water splitting systems at an industrial scale, which first need us to search and develop efficient photocatalysts and suitable reductive/oxidative cocatalysts.
Among all the developed photocatalysts, graphitic carbon nitride (g-C3N4) as a metal-free photocatalyst has captured increasing attention largely due to its appealing properties of availability, low-cost and stability, fulfilling the basic requirements for large-scale industrial synthesis. However, its photocatalytic efficiency is rather low, mainly suffering from the limited efficiencies of the two primary processes in photocatalysis: charge carrier separation and surface catalytic redox reactions.
In a recent article published in Science Bulletin, Prof. Shaohua Shen's research group described an efficient photocatalytic hydrogen production system designed to promote both charge carrier separation and surface catalytic redox reaction processes in g-C3N4.
In their study, g-C3N4 was loaded with ferrite (CoFe2O4 or NiFe2O4), which not only formed Type II band alignment with g-C3N4 to facilitate charge carrier separation, but also accelerated the surface electrocatalytic oxidative reaction kinetics. CoFe2O4 was further demonstrated to be a better modifier for g-C3N4 as compared to NiFe2O4, due to the more efficient charge carrier transfer as well as superior surface oxidative catalytic activity. When loading CoFe2O4 together with reductive hydrogen production electrocatalyst Pt onto g-C3N4, the obtained Pt/g-C3N4/CoFe2O4 photocatalyst achieved visible light (λ > 420 nm) hydrogen production rate 3.5 times as high as Pt/g-C3N4, with the apparent quantum yield achieving 3.35 percent at 420 nm.
This study revealed that creating heterojunctions with synergistically promoted charge carrier separation and accelerated surface catalytic oxidative reaction kinetics would significantly contribute to the photocatalytic hydrogen production performance, which might provide an alternative method for optimizing the semiconductor-based heterostructures for efficient solar fuel production.
More information: Jie Chen et al. Ferrites boosting photocatalytic hydrogen evolution over graphitic carbon nitride: a case study of (Co, Ni)Fe2O4 modification, Science Bulletin (2016). DOI: 10.1007/s11434-016-0995-0
Provided by Science China Press