Credit: Guodong Feng, et al.
(Phys.org)—Zeolite is a Greek word that literally means a boiling stone. They are porous materials used as adsorbents, catalysts, and as ion-exchangers, and used for water filtration, detergents, and air separation, among other things. Typically zeolites are made in the laboratory using a sol-gel process that involves baking the alumino silicate mixture in an oven for several days. While the crystallization process involves bond formation, breakage, and re-formation, the details of this mechanism are largely unknown.
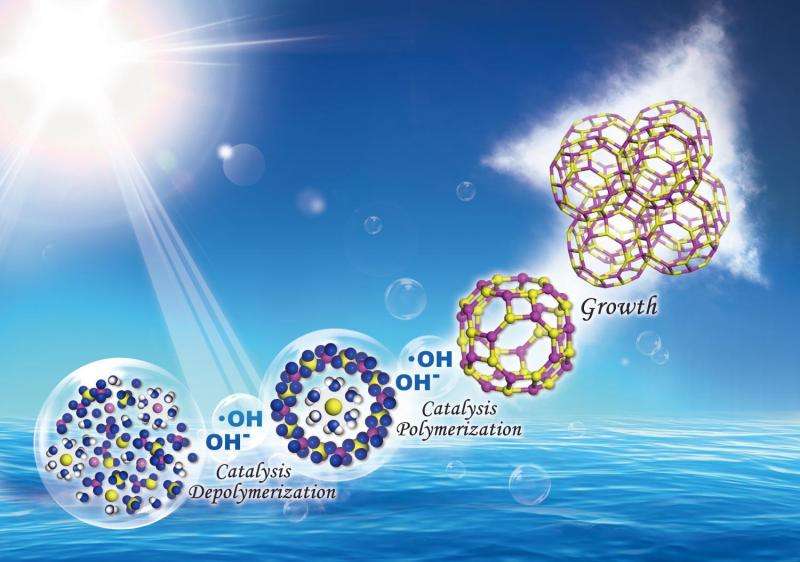
Researchers from Jilin University in China have elucidated a part of the mechanism of zeolite crystallization and have demonstrated that forming hydroxyl radicals (•OH) using UV irradiation produces highly crystalline zeolites in less time than typical syntheses because it speeds up the nucleation process. Their work appears in Science.
Upon baking an alumino-silicate species, in varying proportions in a strong base and in the presence of a cation, researchers are able to make many types of zeolite structures. Feng, et al. wanted to see how the formation of hydroxyl radicals would affect zeolite synthesis. They first compared Na2O-Al2O3-SiO2-H2O systems made under UV conditions and under typical conditions, or what they call "dark conditions."
XRD and SEM studies showed that Na-X, NaZ-21, and Na-A were formed after twenty-four hours under UV conditions, but were still amorphous under dark conditions.
They then wanted to test the effects of different densities of UV irradiation on the system. They used zeolite Na-A at 298K and tested it with UV irradiation of 2.0, 4.0, and 8.0 mW/cm2 as well as under dark conditions. They observed a defined crystal after 52, 40, and 36 hours, respectively.
They then tested the effects of using a smaller molar ratio of Na2O/SiO2 (3.08 vs 4.4). This should reduce the concentration of OH- and would provide further insight into the mechanism. Under dark conditions crystals were formed after 55 hours. Under UV conditions, crystals were formed after 40 hours, which is faster than the higher molar ratio under dark conditions.
Electron paramagnetic resonance (EPR) spectroscopy was used to identify the radical species in both the light and dark conditions, confirming the presence of hydroxyl radicals in the UV conditions. Additional tests showed that hydroxyl radicals are present in zeolites formed from the dark conditions but in lower concentrations.
Another way to form hydroxyl radical is to use Fenton's reagent, a strong oxidant. Using silicalite-1 as an example, Feng, et al. found that Fenton's reagent makes a higher concentration of hydroxyl radicals than UV irradiation. Long-range order of silicalite-1was observed after 40 hours, compared to 50 hours after UV irradiation and 70 hours under dark conditions. This provides further proof that the concentration of hydroxyl radicals affects the rate of zeolite crystallization.
Finally, Feng, et al. tested whether the presence of hydroxyl radicals speeds up both nucleation and crystal growth, or if it only affects one of these steps. By pretreating zeolite Na-A with UV irradiation and then allowing crystallization under dark conditions, they determined that hydroxyl radicals likely speed up the nucleation stage. The crystal growth stage remained constant under both UV and dark conditions.
This research provides valuable insight into the zeolite crystallization mechanism, which can facilitate a more efficient production process in zeolite synthesis.
More information: G. Feng et al. Accelerated crystallization of zeolites via hydroxyl free radicals, Science (2016). DOI: 10.1126/science.aaf1559
Abstract
In the hydrothermal crystallization of zeolites from basic media, hydroxide ions (OH–) catalyze the depolymerization of the aluminosilicate gel by breaking the Si,Al–O–Si,Al bonds and catalyze the polymerization of the aluminosilicate anions around the hydrated cation species by remaking the Si,Al–O–Si,Al bonds. We report that hydroxyl free radicals (•OH) are involved in the zeolite crystallization under hydrothermal conditions. The crystallization processes of zeolites—such as Na–A, Na–X, NaZ–21, and silicalite-1—can be accelerated with hydroxyl free radicals generated by ultraviolet irradiation or Fenton's reagent.
Journal information: Science
© 2016 Phys.org