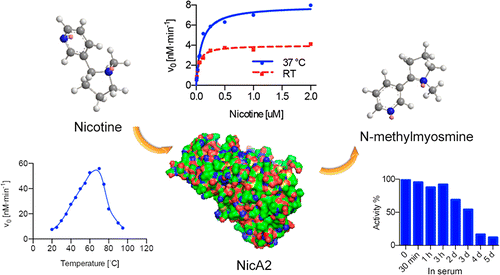
Most people who smoke cigarettes know it's bad for their health, but quitting is notoriously difficult. To make it easier, scientists are taking a brand-new approach. They are turning to bacteria that thrive on nicotine, the addictive component in tobacco. In ACS' Journal of the American Chemical Society, they report successful tests on a bacterial enzyme that breaks down nicotine and could potentially dull its effects in humans.
Tobacco use remains the leading cause of preventable disease, disability and death in the U.S. Smokers who want to quit can turn to various pharmacological aids. These include patches, gum and other nicotine-releasing products designed to replace cigarettes, as well as drugs that sequester nicotine in the body to prevent it from reaching the brain, where its addictiveness takes hold. But the success rates of these options are low. Only about 15 to 30 percent of smokers who try them are able to stop smoking for longer than one year. Kim D. Janda and colleagues wanted to try a new angle.
The researchers used an enzyme called NicA2 that comes from Pseudomonas putida, a kind of bacteria already known to degrade tobacco waste. In lab tests, NicA2 broke down all the nicotine in blood samples within 30 minutes. It also remained stable for more than three weeks in a buffer solution, at least three days in serum, and mice given the enzyme showed no observable side effects.
More information: A New Strategy for Smoking Cessation: Characterization of a Bacterial Enzyme for the Degradation of Nicotine, J. Am. Chem. Soc., Article ASAP. DOI: 10.1021/jacs.5b06605
Abstract
Smoking is the leading cause of preventable diseases; thus, effective smoking cessation aids are crucial for reducing the prevalence of cigarette smoking and smoking-related illnesses. In our current campaign we offer a nicotine-degrading enzyme from Pseudomonas putida, NicA2, a flavin-containing protein. To explore its potential, a kinetic evaluation of the enzyme was conducted, which included determination of Km, kcat, buffer/serum half-life, and thermostability. Additionally, the catabolism profile of NicA2 was elucidated to assess the potential toxicity of the nicotine-derived products. In characterizing the enzyme, a favorable biochemical profile of the enzyme was discovered, making NicA2 a prospective therapeutic candidate. This approach provides a new avenue for the field of nicotine addiction therapy.
Journal information: Journal of the American Chemical Society
Provided by American Chemical Society