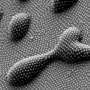
(Phys.org)—UCLA researchers, working in collaboration with colleagues at the University of Washington and Pennsylvania State University have used surface photochemical reactions to probe the critical role of substrate morphology on self-assembly and ligand environment, determining that molecules on curved surfaces have a greater range of orientations and, as a result, react more slowly than do molecules on flat surfaces.
Although researchers have developed extensive strategies for placing and patterning individual molecules, pairs of molecules, lines of molecules and clusters of molecules on flat surfaces, they had not previously been able to confirm whether these same strategies apply to curved and faceted surfaces, such as nanoparticles, nanorods and porous materials. Molecules in solution are free to rotate and thus react differently than do molecules on surfaces, which are held upright and next to each other.
In the present research, the authors investigated how loosely pairs of molecules were held on curved versus flat surfaces by using a novel method of placing proximate pairs of identical reactants on the various surfaces. They found that molecules on curved surfaces do not have enough freedom to tumble around like molecules in solution; however, they have a greater range of orientations and thus react more slowly than do molecules on flat surfaces, presumably because they are not held as tightly.
"This is important because in order to have multifunctional nanoparticles, we have to put different molecules on the nanoparticles, and we need to know how and how many of each molecule attach, and how they are arranged," said study author Paul S. Weiss of UCLA.
The study appears in the journal Nano Letters.
More information: DOI: 10.1021/nl302750d
Journal information: Nano Letters
Provided by University of California, Los Angeles