Image credit: ACS
(PhysOrg.com) -- A team of researchers, led by Dr. Yi Cui, of Stanford and Dr. Bruce Logan from Penn State University have succeeded in developing an entropy battery that pulls energy from the imbalance of salinity in fresh water and seawater. Their paper, published in Nano Letters, describes a deceptively simple process whereby an entropy battery is used to capture the energy that is naturally released when river water flows into the sea.
Up to now, this kind of process has been accomplished by passing seawater though a membrane which unfortunately is too costly to merit creating large-scale operations.
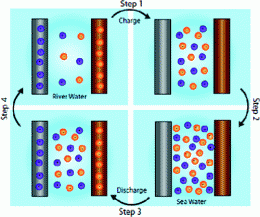
The new process works like this:
Step 1 - Two types of nanorod electrodes are placed in river water; one silver anionic electrode contains Cl- ions and one manganese dioxide cationic electrode contains Na+ ions. The battery charges as the river water’s low salinity concentration of salt pulls the chorine and the sodium from the respective electrodes.
Step 2 - The river water is slowly replaced with seawater, causing a potential difference between the two concentrations of ions in the combined water. This is due to the Cl- ions, or anions, traveling to the silver electrode and the Na+ sodium ions, or cations, traveling to the manganese dioxide electrode.
Step 3 - Ions in the electrodes discharge into the seawater when the electrodes receive more ions than they can accommodate.
Step 4 - The salt water is slowly replaced with river water. This lessens the potential difference of the two electrodes which charges the battery. More energy was released in Step3 into the saltwater than is needed to charge the battery, thus the battery collects and stores the energy that has been building up as the ions have been moving in and out of the crystal lattice of the electrodes.
With the entropy battery, costs are much lower than other ways of accomplishing the same thing due to the absence of replaceable membranes.
Cui believes that the entropy battery might eventually contribute up to 13% of total energy needs. He also believes that by moving the two electrodes closer, he might be able to improve his efficiency rate from 74% percent to 85%.
Because the entropy battery operates in both warm and cold conditions it is a completely renewable resource; one that might lead to mass energy production in both developed countries and those in the third world.
More information: Batteries for Efficient Energy Extraction from a Water Salinity Difference, by Fabio La Mantia et al., Nano Lett., Article ASAP, Publication Date (Web): March 17, 2011. pubs.acs.org/doi/abs/10.1021/nl200500s
© 2010 PhysOrg.com