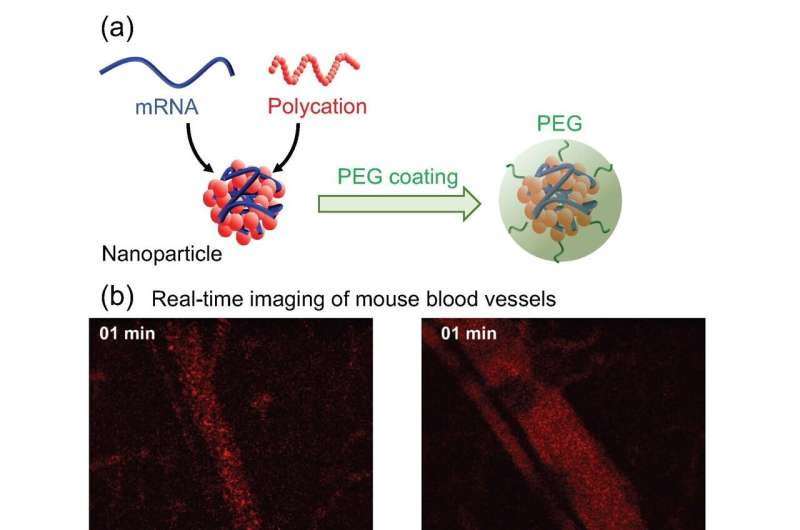
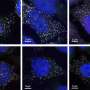
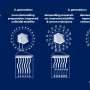
(a) Preparation scheme. (b) Behavior in the blood circulation. Credit: Department of Advanced Nanomedical Engineering, TMDU
Medicine can help to treat certain illnesses; for example, antibiotics can help overcome infections, but a new, promising field of medicine involves providing our body with the "blueprint" for how to defeat illnesses on its own.
mRNA therapeutics is the delivery of messenger RNA (mRNA) molecules into the body, which the cellular machinery can use to make specific proteins. The field is rapidly advancing, especially because mRNA vaccines have proven successful against COVID-19. However, the delivery of these engineered mRNAs to a specific organ has proved challenging.
Now, a team at Tokyo Medical and Dental University (TMDU) has shown that coating the engineered mRNAs with a molecule called polyethylene glycol, or PEG, allows their delivery selectively to the spleen. The research is published in the journal Small Science.
Engineered mRNAs have been packaged into structures called polyplexes for delivery into the body. The polyplex structures allow mRNAs to remain stable while outside cells and to be released in a controlled manner once inside cells. Once inside, the mRNAs are used by cellular machinery to produce proteins that are naturally dysfunctional or absent.
Without modification, the polyplexes tend to accumulate in the lungs, as after injection into the blood, they rapidly stick to each other and surrounding proteins and cells and become lodged in the lung's blood vessels. Treating polyplexes with PEG, a process called "PEGylation," prevents them from sticking together; however, applying PEG in a controlled, consistent manner to the polyplex surface is very difficult.
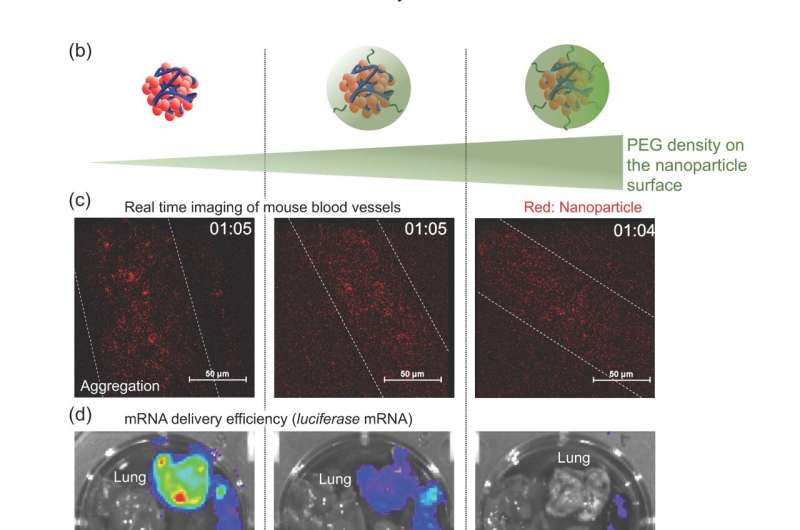
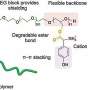
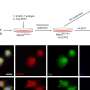
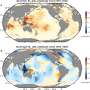
(a) Preparation scheme. (b) Nanoparticles with varying PEG density on the surface. (c) Behavior in the blood circulation. (d) Delivery of luciferase mRNA. Credit: Department of Advanced Nanomedical Engineering, TMDU
The team at TMDU has developed a new method of PEGylation, where the mRNAs are hybridized to PEG molecules before the polyplexes are formed. Using this method, almost all the PEG strands mixed into the reaction become bound to the polyplexes, allowing much greater control over the final amount of PEG on the polyplex surface.
Using a mouse model, the team found that the quantities and lengths of the PEG molecules significantly affected how well the mRNA therapy worked. A small number of short PEG molecules prevented the accumulation of the engineered mRNAs in the lungs, facilitating effective delivery to the spleen. This approach has demonstrated utility in mRNA vaccines.
"Our novel method allows fine-tuning of the amount of PEGylation of mRNA polyplexes," explains senior author Dr. Satoshi Uchida, "which in turn allows control of the physicochemical properties of the polyplexes, and thus their biological functionalities."
mRNA technology has wide-ranging potential for treating many diseases that have previously been considered incurable, as well as for the development of novel cancer treatments and vaccines. The development of this innovative technique paves the way for significant advances in the therapeutic use of mRNA polyplexes, with far-reaching potential consequences for human health.
More information: Miki Suzuki et al, Poly(ethylene Glycol) (PEG)–OligoRNA Hybridization to mRNA Enables Fine‐Tuned Polyplex PEGylation for Spleen‐Targeted mRNA Delivery, Small Science (2024). DOI: 10.1002/smsc.202300258
Provided by Tokyo Medical and Dental University