August 18, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Sniffing nanoparticles loaded with mRNA could lead to advanced lung therapeutics
Researchers at Yale University, New Haven, have optimized a polymer-based mRNA vehicle for targeted lung delivery and demonstrated the potential of the platform for mucosal vaccination against respiratory pathogens.
In a paper, "Polymer nanoparticles deliver mRNA to the lung for mucosal vaccination," published in Science Translational Medicine, the team introduces their creation of inhalable messenger RNA (mRNA) for therapeutic use.
Clinical research has been searching for an efficient and targeted way to deliver mRNA to the lungs for various therapeutic applications, including protein replacement therapies, gene editing and vaccination. The main challenges have been maintaining mRNA stability and avoiding immune interference.
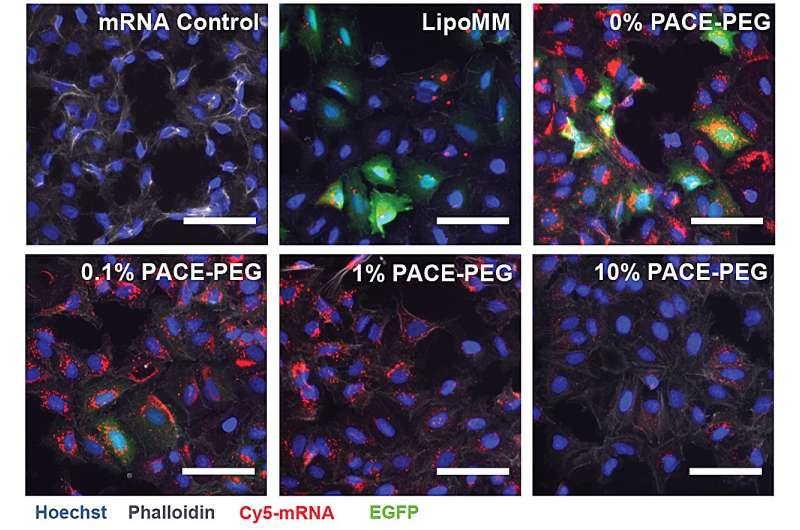
The Yale team created PACE (Polymerized Albumin Conjugates for mRNA Encapsulation) polymer formulations to deliver local mRNA to the lungs. The researchers optimized PACE polyplexes to enhance mRNA protection, transfection efficiency, and antigen presentation for effective lung-specific therapeutic and vaccination strategies.
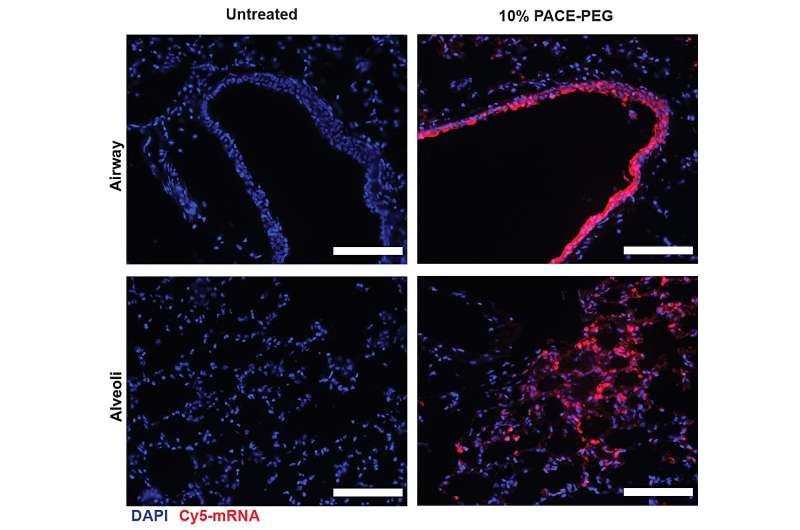
To stabilize PACE, an optimized ratio of polyethylene glycol (PEG) molecules were integrated into the polymer structure during the enzymatic copolymerization process, which stabilized the polyplexes and modified key characteristics. PEG was able to affect the size, surface charge, and other properties of the polyplexes, making them more suitable for loading and effective at mRNA delivery to lung cells.
The stabilized formulation performed poorly in an in vitro cell culture experiment. The researchers note that traditional cell culture methods are not good predictors of delivery systems, either positively or negatively. The environment within the body, especially the lungs, interacts very differently than a collection of cells. For instance, the mucosal surfaces are missing, and those surfaces are exactly what the PACE-PEG system is designed to take advantage of. The real test would come in vivo with a mouse model.
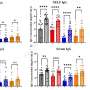
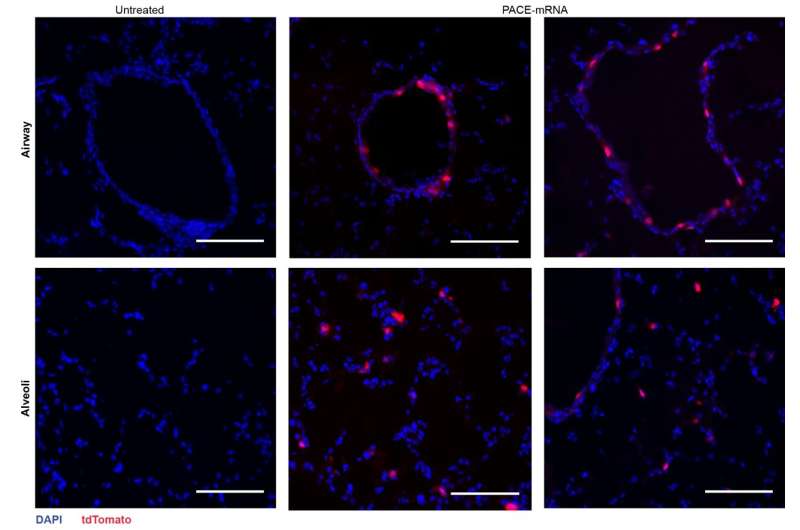
The researchers encapsulated mRNA encoding the spike protein from SARS-CoV-2 into PACE and inoculated mice susceptible to SARS-CoV-2 infection. Mice received a 10-μg dose of PACE-mRNA delivered intranasally on days 0 and 28. The development of adaptive immunity in the mediastinal lymph nodes was tested and confirmed 14 days after the boost.
After assessing the local immune response, the researchers examined lung tissues, blood serum, and bronchoalveolar lavage fluid for local and systemic antigen-specific T-cell and antibody responses. Transfection occurred primarily in lung epithelial cells and antigen-presenting cells, two cell types that are relevant targets for pulmonary diseases
The vaccination successfully increased spike protein-specific CD8+ T cells in the lung tissue and circulating CD8+ T cells in the bloodstream. CD8+ T cells expressed markers indicative of tissue-resident memory. Both circulating and mucosal IgG antibodies were found at significantly higher concentrations in vaccinated mice.
Mice were then introduced to a lethal dose of SARS-CoV-2. PACE-mRNA vaccination significantly reduced the viral burden in the lungs and improved the weight and survival of the vaccinated mice. This protection was attributed to the spike protein-specific immune response induced by the vaccination.
The control group showed no evidence of a spike protein-specific immune response and did not exhibit reduced viral load or improved survival after the viral challenge.
The study presents PACE-mRNA polyplexes as a promising method for efficient and targeted mRNA delivery to the lungs with potential benefits for both therapeutic protein expression and mucosal vaccination against respiratory pathogens.
The study also illustrates the importance of animal models as opposed to cell culture alone in determining real-world effects. The positive results indicate that more research is warranted, with further testing planned on larger animal models.
More information: Alexandra Suberi et al, Polymer nanoparticles deliver mRNA to the lung for mucosal vaccination, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.abq0603
Journal information: Science Translational Medicine
© 2023 Science X Network