This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Using hybrid nanotubes to enhance cancer treatment with intracellular protein delivery
The intracellular delivery of proteins is an important technique for unveiling the cellular functions, protein complex structure, and therapeutics. However, conventional delivery methods have several limitations.
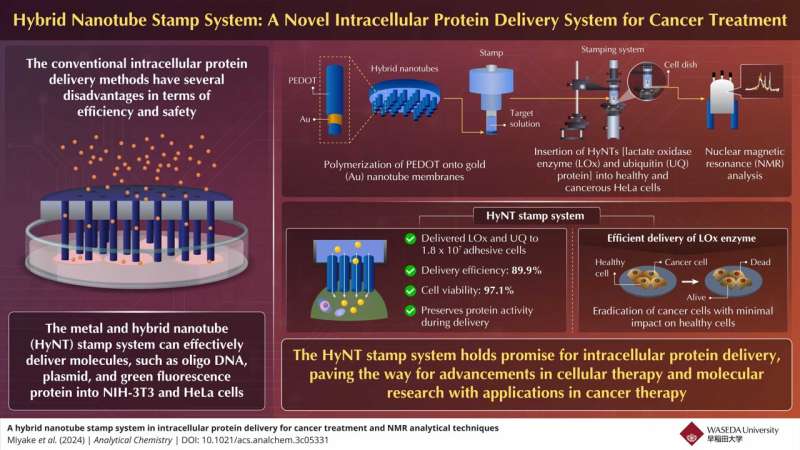
To address this, researchers from Japan have developed a novel hybrid nanotube (HyNT) stamp system that can deliver multiple proteins with high efficiency and viability rates. This system represents an advancement in intracellular protein delivery, offering precise injection of therapeutic agents into target cells.
In today's medical landscape, precision medicine and targeted therapies are gaining traction for their ability to tailor treatments to individual patients while minimizing adverse effects.
Conventional methods, such as gene transfer techniques, show promise in delivering therapeutic genes directly to cells to address various diseases. However, these methods face significant drawbacks, hindering their efficacy and safety.
Intracellular protein delivery offers a promising approach for developing safer, more targeted, and effective therapies. By directly transferring proteins into target cells, this method circumvents issues such as silencing during transcription and translation and the risk of undesirable mutations from DNA insertion. Additionally, intracellular protein delivery allows for precise distribution of therapeutic proteins within target cells without causing toxicity.
A group of researchers led by Professor Takeo Miyake at Waseda University, Japan, in collaboration with the Mikawa Group at the RIKEN Institute, have now developed a hybrid nanotube stamp system for intracellular delivery of proteins. This innovative technique enables the simultaneous delivery of diverse cargoes, including calcein dye, lactate oxidase (LOx) enzyme, and ubiquitin (UQ) protein, directly into adhesive cells for cancer treatment.
An article describing the research is published in Analytical Chemistry. This article has been co-authored by Dr. Tsutomu Mikawa, Dr. Masaomi Ikari, Dr. Hiromasa Yagi, Dr. Naoya Tochio, and Dr. Takanori Kigawa from RIKEN Center for Biosystems Dynamics Research, Japan and Mr. Bowen Zhang, Mr. Bingfu Liu, Mr. Zhouji Wu, and Mr. Kazuhiro Oyama from Waseda University, Japan.
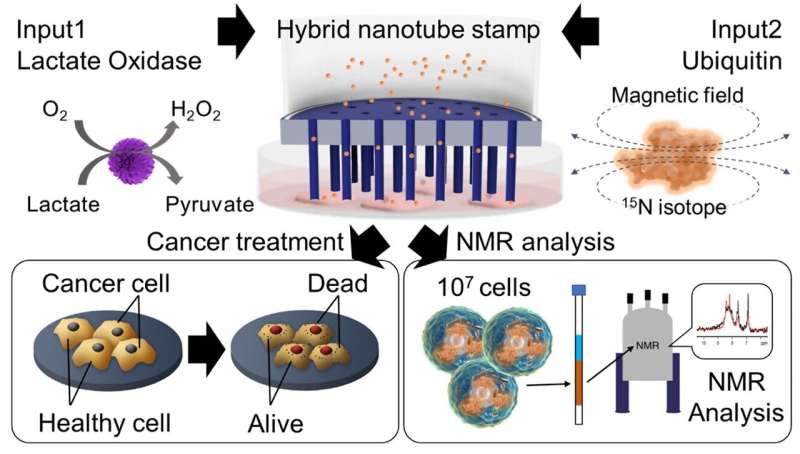
Miyake briefly explains the stamp system assembly. "The HyNTs were synthesized through PEDOT polymerization onto Au nanotube membranes, and then assembled with a glass tube to create a stamp capable of physically inserting HyNTs into cells."
The researchers explored the therapeutic potential of delivering LOx enzyme for cancer treatment. "Through our innovative stamp system, we successfully delivered LOx into both healthy mesenchymal stem cells (MSC) and cancerous HeLa cells. While MSC cells remained unaffected, we observed significant cell death in HeLa cancer cells following LOx treatment with viabilities decreasing over time.
"Our findings highlight the promising efficacy of intracellularly delivered LOx in selectively targeting and killing cancer cells, while sparing healthy cells, offering a targeted therapeutic strategy for cancer treatment," explains Miyake.
Finally, the team successfully delivered 15N isotope-labeled UQ proteins into HeLa cells using the HyNT stamp system. This delivery allowed for the analysis of complex protein structures and interactions within the cells.
In addition, optical and fluorescence imaging confirmed the presence of delivered UQ in HeLa cells, and nuclear magnetic resonance spectroscopy matched the intracellular UQ protein concentration with that of a solution containing 15N-labeled UQ.
These results demonstrate the effectiveness of the stamp system in delivering target proteins for subsequent analysis.
The results demonstrate the remarkable capability of the HyNT stamp system in delivering LOx and UQ into a substantial number of adhesive cells, as required for regenerative medicine applications.
The system achieved a notably high delivery efficiency of 89.9%, indicating its effectiveness in transporting therapeutic proteins into the target cells with precision. Moreover, the cell viability rate of 97.1% highlights the system's ability to maintain the health and integrity of the treated cells throughout the delivery process.
The HyNT stamp system offers transformative potential in intracellular protein delivery, with applications spanning from cancer treatment to molecular analysis. Beyond medicine, its versatility extends to agriculture and food industries, promising advancements in crop production and food product development.
With precise cell manipulation and efficient delivery, the HyNT stamp system is poised to revolutionize biomedical research, clinical practice, and diverse industries, paving the way for personalized interventions and shaping the future of modern medicine.
More information: Bowen Zhang et al, A Hybrid Nanotube Stamp System in Intracellular Protein Delivery for Cancer Treatment and NMR Analytical Techniques, Analytical Chemistry (2024). DOI: 10.1021/acs.analchem.3c05331
Journal information: Analytical Chemistry
Provided by Waseda University