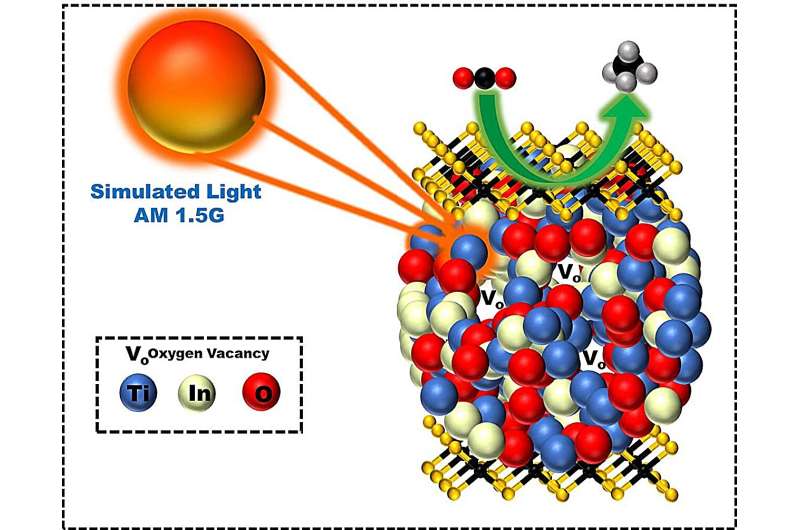
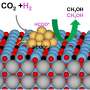
Graphical abstract. Credit: Chemical Engineering Journal (2023). DOI: 10.1016/j.cej.2023.147966
DGIST Professor In Su-il's research team has developed a high-efficiency photocatalyst that utilizes sunlight to convert carbon dioxide (CO2), the primary cause of global warming, into methane (CH4) fuel. The research team expects that this environmentally friendly technology can be applied to Carbon Capture and Utilization (CCU) technology.
According to a US university research team, the current concentration of carbon dioxide in the atmosphere has reached its highest level in 14 million years, at 420 ppm. The World Meteorological Organization (WMO) predicts that 2024 will be a hotter year than last year due to the influence of El Niño.
The World Economic Forum (WEF) has identified climate change as the greatest global risk among 34 crises faced by the world in fields including economics, society, technology, and geopolitics, which could lead to international conflicts as a result of the depletion of resources and polarization. Therefore, reducing the concentration of carbon dioxide in the atmosphere is inevitable to overcome crises induced by climate change.
In this regard, research on photocatalysts, capable of reducing carbon dioxide emissions while simultaneously converting it into useful fuel, has been actively pursued. Photocatalyst research has garnered attention as a promising Carbon Capture and Utilization (CCU) technology for the future, as they rely solely on sunlight without the need for additional energy input, such as electricity, making their systems inherently simple.
However, most photocatalysts developed so far are composed of a crystal structure with regularly arranged atoms. Researchers have, therefore, faced constraints, such as the conditions for the composition to adhere to the arrangement of constituent elements, in designing various active spots within the catalyst while maintaining the structure.
Against this backdrop, Professor In Su-il's research team at DGIST has developed a high-efficiency photocatalyst that includes various active spots and improves electron transfer performance.
The research team fabricated an "amorphous structure of In2TiO5 photocatalyst" containing "Ti3+ active spots that can adsorb and activate carbon dioxide" and "In3+ active spots that can decompose water to supply protons," and incorporated it into molybdenum diselenide (MoSe2) nanolayers to improve electron transfer performance.
Through structural analysis, the research team confirmed that the newly developed photocatalyst converts methane 51 times more than the commercially available TiO2 photocatalysts.
Professor In Su-il of DGIST said, "This research holds significance as it has developed a high-efficiency photocatalyst technology featuring dual active spots. We will conduct follow-up research on improving energy loss and stability of amorphous photocatalysts for future commercialization of the technology."
The research is published in the Chemical Engineering Journal.
More information: Niket S. Powar et al, Dynamic Ti3+ and In3+ dual active sites on In2TiO5 to enhance visible-light-driven gas-phase photocatalytic CO2 reduction, Chemical Engineering Journal (2023). DOI: 10.1016/j.cej.2023.147966
Journal information: Chemical Engineering Journal
Provided by DGIST (Daegu Gyeongbuk Institute of Science and Technology)