Highly efficient photocatalyst that converts carbon dioxide to methane
A research team of Energy Science and Engineering at DGIST has developed a titanium dioxide (TiO2)-based, high-efficiency photocatalyst that converts carbon dioxide to methane using a simple reduction method.
The photocatalysts developed by the research team can be used to convert carbon dioxide to fuels like methane. Therefore, it can be applied to technologies for carbon dioxide abatement and resource reclamation.
Anthropogenic emission of greenhouse gases, particularly CO2, is a significant factor driving global climate change; sustainable, low-carbon, readily portable fuels are one of the most pressing needs of modern society. To that end, there has been a worldwide effort underway to find ways to convert carbon dioxide, a major contributor to global warming, into a usable fuel, such as hydrogen, methane, ethanol, methanol and butanol.
In order to use carbon dioxide as a resource, it is essential to increase the conversion efficiency and light absorption efficiency when converting carbon dioxide into fuel, and to use photocatalysts to prevent secondary harmful substances.
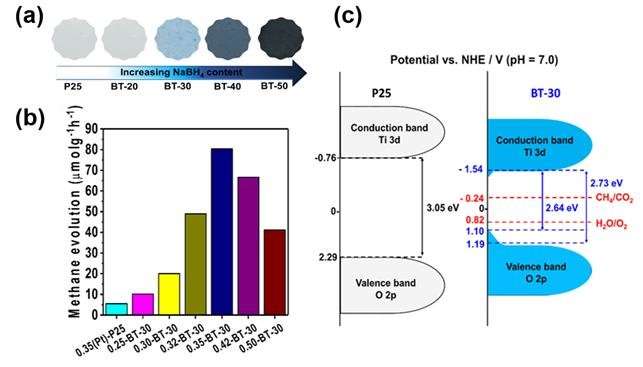
Carbon dioxide recycling depends on synthesizing materials such as titanium dioxide, copper oxide, and reduced graphene oxide, or controlling the structure and surface of photocatalyst material. DGIST's research team has discovered a synthesis method that rapidly reduces titanium dioxide (TiO2) at low temperatures using a strong reducing agent, sodium borohydride (NaBH4).
In the study, titanium dioxide-based photocatalysts using this synthesis method showed 12.49 percent conversion of methane to photochemical carbon dioxide in the gas phase, which represents the highest conversion rate among the introduced photocatalysts so far.
In addition, the photocatalyst developed by the research team exhibits controlled band gap through the conversion of the oxidation number from four to three by breaking the oxygen atoms on the surface of titanium dioxide. This change increases the amount of light absorption and efficiently separates the charge, resulting in higher carbon conversion of carbon dioxide. Moreover, the experiment has also proved that the efficiency of methane conversion of carbon dioxide can be increased up to 29 times using platinum nanoparticles.
Professor In said, "The newly developed titanium dioxide photocatalyst is superior to the other photocatalysts reported so far as it has outstanding carbon dioxide conversion efficiency as well as excellent stability. We would like to contribute to the development of carbon dioxide reduction and recycling technology by conducting further researches to improve conversion efficiency to the extent that it can be commercialized."
More information: Saurav Sorcar et al, Highly enhanced and stable activity of defect-induced titania nanoparticles for solar light-driven CO 2 reduction into CH 4, Materials Today (2017). DOI: 10.1016/j.mattod.2017.09.005
Journal information: Materials Today
Provided by Daegu Gyeongbuk Institute of Science and Technology