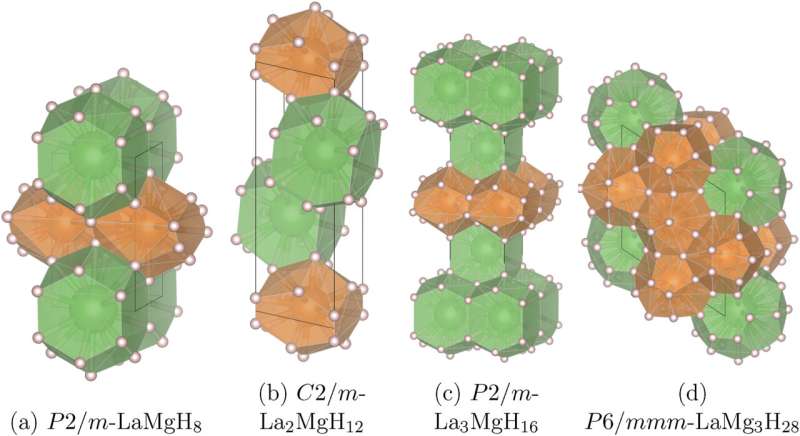
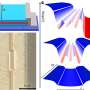
Crystal structures of the lanthanum-magnesium hydrides (left to right) LaMgH8, La2MgH12, La3MgH16, and LaMg3H28. Credit: Materials Today Physics (2023). DOI: 10.1016/j.mtphys.2023.101300
Skoltech researchers and their colleagues from MIPT and China's Center for High Pressure Science and Technology Advanced Research have computationally explored the stability of the bizarre compounds of hydrogen, lanthanum, and magnesium that exist at very high pressures. In addition to matching the various three-element combinations to the conditions at which they are stable, the team discovered five completely new compounds of hydrogen and either magnesium or lanthanum only.
Published in Materials Today Physics, the study is part of the ongoing search for room-temperature superconductors, the discovery of which would have enormous consequences for power engineering, transportation, computers and more.
"In the previously unexplored system of hydrogen, lanthanum, and magnesium, we find LaMg3H28 to be the 'warmest' superconductor. It loses electrical resistance below –109°C, at about 2 million atmospheres—not a record, but not bad at all either," the study's principal investigator, Professor Artem R. Oganov of Skoltech, commented.
"Importantly, though, we also furnish a fresh confirmation of the validity of an empirical rule that guides the search for higher-temperature superconductors. This is the paper's central finding, along with the five new binary compounds, including LaH13 and MgH38. These are highly exotic compositions for which a theoretical explanation is yet to be proposed."
"Moreover, we proposed a new approach for studying very large chemical spaces, and demonstrated its effectiveness for the La–Mg–H system," said Ivan Kruglov, who conducted this study at MIPT.
As for the empirical rule confirmed by the study, it has to do with the transfer of electrons from the metal atoms to the hydrogen atoms. It is believed that what promotes superconductivity is the numerous relatively weak covalent bonds between many hydrogen atoms, connected in a 3D network.
However, a hydrogen atom can capture up to one entire electron from lanthanum or magnesium, turning it into a negative hydride ion that does not seek any further chemical bonds. Alternatively, if hydrogen gets no electrons from the metal atoms, it satisfies that need by forming H2 molecules with other hydrogen atoms.
"It turns out that an average of one-third of an electron per hydrogen atom is the magic number," Oganov said. "The closer to it the better for superconductivity. This has been noted for some time, and our study delivers yet another confirmation, this time on a fairly complex chemical system."
More information: Grigoriy M. Shutov et al, Ternary superconducting hydrides in the La–Mg–H system, Materials Today Physics (2023). DOI: 10.1016/j.mtphys.2023.101300
Provided by Skolkovo Institute of Science and Technology