January 8, 2021 report
Experiments with bifluoride ions show evidence of hybrid bonds
A team of researchers from the University of Chicago and Emory University has found evidence of a hydrogen bond/covalent bond hybrid. In their paper published in the journal Science, the group describes experiments they conducted with bifluoride ions that blurred the line between hydrogen bonds and covalent bonds. Mischa Bonn and Johannes Hunger with the Max Planck Institute for Polymer Research have published a Perspectives piece in the same journal issue outlining the work by the team on this new effort.
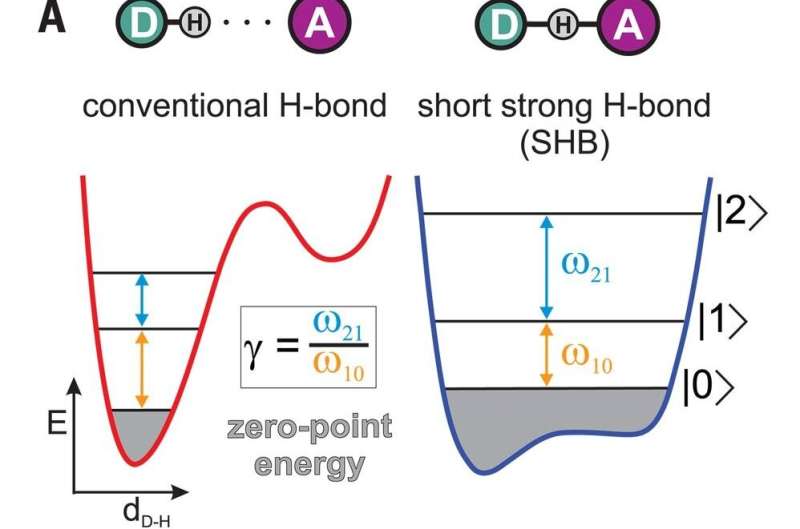
One of the basic understandings of chemistry is that the bonds between hydrogen atoms are electrical and weak, and thus are not true chemical bonds. Covalent bonds, on the other hand, are true chemical bonds and are therefore strong—and they are what typically hold molecules together. Covalent bonds get their strength by sharing electrons between the atoms involved. In this new effort, the researchers have found what appears to be an exception to this rule—a hybrid type of bond.
The researchers were working with groupings of bifluoride ions—each was made by placing a hydrogen atom between two fluorine atoms in a water solution. According to the rules of chemistry, the triplets should have been held together by the hydrogen atom forming a covalent bond with one of the fluorine atoms and a hydrogen bond with the other. But when the researchers tested the triplets using infrared light to make them vibrate, they found something surprising. Instead of the expected decrease between energy levels as the atoms ascended the energy ladder, they found an increase—a sign that the hydrogen atom was being equally shared between the two fluorine atoms. Computer calculations showed that the behavior they had observed depended on how much distance there was between the atoms. It was only when the fluorine atoms were closest together that the hybrid bond was observed. As the fluorine atoms were pulled farther from the hydrogen atom, the normal bonding took over.
The researchers have named the hybrid a hydrogen-mediated chemical bond, and note that it cannot be described as either a hydrogen bond or a covalent bond—it truly is a hybrid of the two. They note also that their findings have implications for the understanding of basic chemistry and the true nature of chemical bonds.
More information: Bogdan Dereka et al. Crossover from hydrogen to chemical bonding, Science (2021). DOI: 10.1126/science.abe1951
Mischa Bonn et al. Between a hydrogen and a covalent bond, Science (2021). DOI: 10.1126/science.abf3543
Journal information: Science
© 2021 Science X Network