March 11, 2020 report
Measuring variations of an atom's chemical reactivity through its chemical bonds
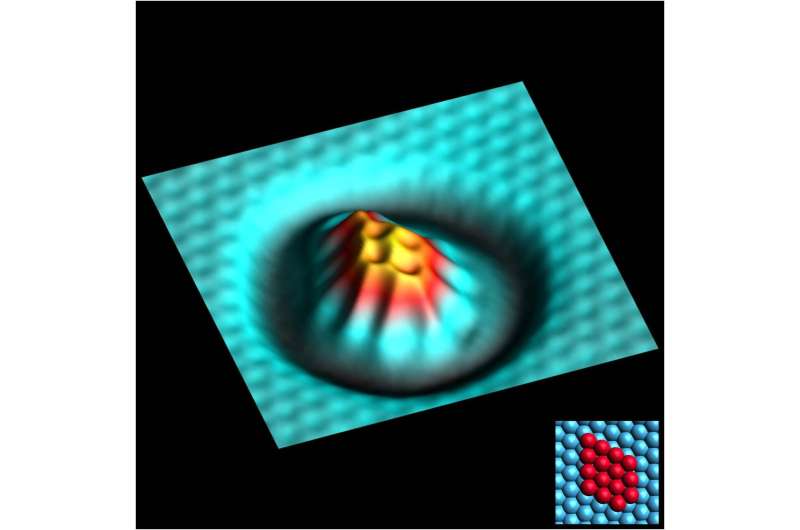
A team of researchers from the University of Regensburg and Ludwig-Maximilians-University Munich has developed a way to measure the dependence of an atom's chemical reactivity on its chemical bonds. In their paper published in the journal Physical Review Letters, the group outlines their process and what they found when it was tested.
Prior research has shown that chemical reactivity is impacted by the degree of atomic isolation of an atom. Those that have several bonds are less apt to bond with other atoms than are atoms with few bonds. This simple concept plays a major role in catalytic and chemical activity. But until now, there has not been a way for chemists to directly and quantitatively measure variations in chemical reactivity of atoms that are bonded with different numbers of other atoms. In this new effort, the researchers have found a way to do it and in a way that is easily reproducible by others.
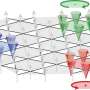
The work involved a type of scanning probe microscopy (SPM) that works without making contact with material under review—a type that also allowed for imaging, manipulating and spectroscopically analyzing single atoms and the bonds between them. The researchers attached a CO molecule to the tip of the SPM probe and then used it to build different size clusters of iron atoms (from 3 to 25)—a means of regulating the amount of coordination with other atoms. They then used the tip to probe the atoms in the cluster—as the tip moved near an atom, bonding occurred, which could be measured. By noting variations in bonding, the team was able to calculate the strength of the bonds that formed between the atoms when they were part of different sized clusters—and that gave a direct measure of the atom's degree of reactivity.
In testing their technique, the researchers found that corner atoms and those in edges of a group were less reactive than others in the middle of a group—a finding that was expected. However, in a new result, the team was able to provide a measurable determination of the chemical reactivity of an atom based on the degree of coordination it had with other atoms.
More information: Julian Berwanger et al. Atomically Resolved Chemical Reactivity of Small Fe Clusters, Physical Review Letters (2020). DOI: 10.1103/PhysRevLett.124.096001
Journal information: Physical Review Letters
© 2020 Science X Network