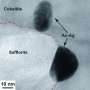
Selected Area Electron Diffraction ring pattern from gold nanoparticles. The rings are indexed to Au as per the Joint Committee on Powder Diffraction Standards (JCPDS) card no. 04-0784. Credit: arXiv (2023). DOI: 10.48550/arxiv.2310.15125
Scientists at the University of Bristol have discovered why fulminating gold—the world's first known high explosive—produces a purple smoke when it detonates, solving a 400-year-old alchemy puzzle.
Fulminating gold was first discovered by alchemists in the 16th century. It is a mixture of a number of different compounds, with ammonia providing the majority of the material's explosive power.
German alchemist Sebald Schwaertzer noted the unusual purple smoke given off when fulminating gold was detonated in 1585, and the material was later studied by leading figures of chemistry in the 17th and 18th centuries, including Robert Hooke and Antoine Lavoisier.
But while the chemistry of the fulminating gold recipe has been understood for centuries, one question remained unanswered—why does its detonation produce purple smoke?
It has long been supposed, yet previously never proven that the rich purple color of this cloud was due to it being formed of gold nanoparticles. This has now changed thanks to Professor Simon Hall, Professor of Chemistry at the University of Bristol, and his Ph.D. student Jan Maurycy Uszko.
"I was delighted that our team have been able to help answer this question and further our understanding of this material," Professor Hall said.
"Our experiment involved creating fulminating gold, then detonating 5mg samples on aluminum foil by heating it. We captured the smoke using copper meshes and then analyzed the smoke sample under a transmission electron microscope," Professor Hall explained.
"Sure enough, we found the smoke contained spherical gold nanoparticles, confirming the theory that the gold was playing a role in the mysterious smoke."
Having solved one historic scientific puzzle, Professor Hall and his team plan to use this methodology to study the precise nature of clouds produced by other metal fulminates such as platinum, silver, lead, and mercury, which remain an open question.
The paper, "Explosive Chrysopoeia," is available on the arXiv preprint server
More information: Jan Maurycy Uszko et al, Explosive Chrysopoeia, arXiv (2023). DOI: 10.48550/arxiv.2310.15125
Journal information: arXiv
Provided by University of Bristol