This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers develop increasingly sustainable methods for dissolving gold, silver and copper from recycled materials

Waste from computers and cell phones, solar panels and other discarded electronics are becoming an important source of noble metals alongside mining. Researchers at the University of Helsinki have developed sustainable dissolution methods for noble metals.
The extraction methods currently in use consume a lot of energy and are detrimental to the environment. The method of roasting is particularly dangerous for its practitioners and the environment because it releases hazardous chemicals. In developing countries, noble metals are to this day extracted under crude conditions in landfills. Even though advanced hydrometallurgical processes are safer and able to dissolve noble metals, the result is metal mixtures that require further processing.
The findings of the Catalysis and Green Chemistry research group headed by Professor of Chemistry Timo Repo have been published in the journal Angewandte Chemie International Edition.
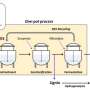
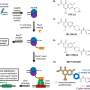
The article introduces a three-stage process where copper is first dissolved from electronic waste, followed by silver and, finally, gold. This way, metals can be selectively separated from plastic, ceramics and other materials, yielding pure noble metals. In addition, the solvents used can be easily recycled.
Researchers at the University of Helsinki tested organic solvents on crushed circuit boards, successfully extracting the gold and copper contained in them. Silver was separated from crushed old solar panels. This result is particularly interesting because solar panels are a high-volume product whose recycling has thus far been extremely challenging.
"In this study, we used what are known as deep eutectic solvents, liquids, that are made from substances that are solid in room temperature and under normal pressure, such as choline chloride—also used in poultry feed—and urea, as well as other safe organic compounds," says Postdoctoral Researcher Anže Zupanc from the Department of Chemistry, University of Helsinki.
Deep eutectic solvents are a special type of solvent composed of two or more simple compounds, which together form a mixture with a low melting point. These solvents are known as deep eutectic, as their melting point is considerably lower than the melting point of each component on its own.
Deep eutectic solvents are environmentally friendly, renewable and in many cases biodegradable. They have many applications as solvents, including in chemical reactions, catalysis and extraction techniques.
In this study, lactic acid and hydrogen peroxide were used as solvents as well.
"An important result was that the solvents could be reused, putting the principles of green chemistry into practice," Professor Repo notes.
According to Repo, the results obtained in laboratory conditions constitute a significant step towards sustainable chemical processes.
More information: Anže Zupanc et al, Sequential Selective Dissolution of Coinage Metals in Recyclable Ionic Media, Angewandte Chemie International Edition (2024). DOI: 10.1002/anie.202407147
Journal information: Angewandte Chemie International Edition
Provided by University of Helsinki