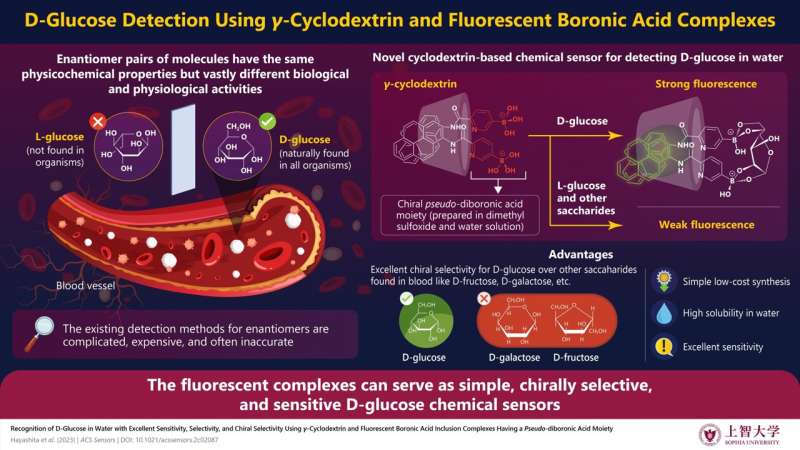
Researchers from Japan have developed γ‑cyclodextrin and fluorescent monoboronic acid-based complexes—1F/γ-CyD and 2N/γ-CyD—that exhibit excellent sensitivity, selectivity, and chiral selectivity for D-glucose detection. Credit: Takashi Hayashita/Sophia University
Diabetes mellitus, simply called diabetes, is a metabolic disorder characterized by the presence of abnormally high concentrations of glucose in blood. Existing methods for the diagnosis of diabetes rely on traditional techniques of detecting glucose in blood serum samples—a process that is typically tedious and expensive.
Molecular recognition is the science of accurately detecting specific compounds by exploiting their binding properties. Here, a receptor molecule—a kind of sensor—selectively binds to a target molecule. This process triggers some reaction, say, a change in fluorescence. As a result, the target gets detected. Chemical sensors, specialized polymers, and some catalysis techniques work on this principle.
Despite advancements in molecular recognition across decades, developing receptors for detecting chiral (or asymmetric) molecules remains a challenge. Chirality results in enantiomeric pairs, which are non-superimposable "mirror images" of the same molecule. They have identical physical and chemical properties but different biological functions. Their similar structures make them hard to distinguish from each other. Therefore, researchers must employ complex and expensive techniques, such as high-performance liquid chromatography, to tell them apart.
In this light, a group of researchers, including Professor Takashi Hayashita and Dr. Yota Suzuki of the Department of Materials and Life Sciences at Sophia University, has designed an all-new fluorescence recognition method for detecting D-glucose, a chiral monosaccharide, in water. Their work was made available online on December 20, 2022 and published in ACS Sensors on January 27, 2023.
Dr. Hayashita describes the motivation behind the research: "Most approaches for designing D-glucose chemical sensors require complicated syntheses, often have poor solubility in water, and sometimes have poor selectivity. Therefore, a novel detection mechanism has been developed."
The researchers developed a complex consisting of γ-cyclodextrin (γ-CyD), which has a cavity that provides a hydrophobic microenvironment to encapsulate hydrophobic compounds spontaneously in an aqueous environment. They then readily synthesized two types of simple hydrophobic fluorescent monoboronic acid-based receptors: a 3-fluorophenylboronic acid-based receptor (1F) and a pyridyl boronic acid-based receptor (2N). They attached two molecules of either receptor to γ-CyD.
The resulting inclusion complexes (1F/γ-CyD or 2N/γ-CyD) formed a pseudo-diboronic acid moiety that selectively recognized D-glucose in water at its two sites. This strongly enhanced the fluorescence of the solution. In contrast, only weak fluorescence was observed for nine other saccharides that were tested including D-fructose, D-galactose, and D-mannose, which were typical saccharides contained in the blood. 1F/γ-CyD and 2N/γ-CyD increased the fluorescence by 2.0 and 6.3 times, respectively, for D-glucose, relative to its enantiomer L-glucose.
"To the best of our knowledge, 2N/γ-CyD has the highest D/L selectivity among other reported fluorescent diboronic acid molecule-based receptors," says Dr. Suzuki.
The researchers investigated this phenomenon further through induced circular dichroism spectral and nuclear magnetic resonance studies. They found that a D-glucose molecule bridges the two monoboronic acid molecules. It rigidifies the complex structure and enhances the fluorescence. In the case of non-glucose saccharides, two different molecules bind to the two sites of the pseudo-diboronic acid moiety. As a result, the fluorescence remains weak.
Besides high selectivity, the developed complexes also show remarkable sensitivity. 1F/γ-CyD and 2N/γ-CyD could detect D-glucose concentrations with low limits of detection (LODs) of 1.1 μM and 1.8 μM, respectively. Hence, both complexes can serve as simple D-glucose chemical sensors. They have excellent selectivity, sensitivity, and chiral selectivity.
"The developed fluorescent sensors are useful for detecting D-glucose selectively and discriminating glucose enantiomers. They can potentially also serve as next-generation diagnosing systems for diabetes that can be used with minute amounts of blood sample, which is an indispensable feature when drawing blood from infants. Since their chemical structures are quite simple, these sensors will help develop affordable and reproducible kits for its early diagnosis," concludes Dr. Hayashita.
More information: Yota Suzuki et al, Recognition of d-Glucose in Water with Excellent Sensitivity, Selectivity, and Chiral Selectivity Using γ-Cyclodextrin and Fluorescent Boronic Acid Inclusion Complexes Having a Pseudo-diboronic Acid Moiety, ACS Sensors (2022). DOI: 10.1021/acssensors.2c02087
Journal information: ACS Sensors
Provided by Sophia University