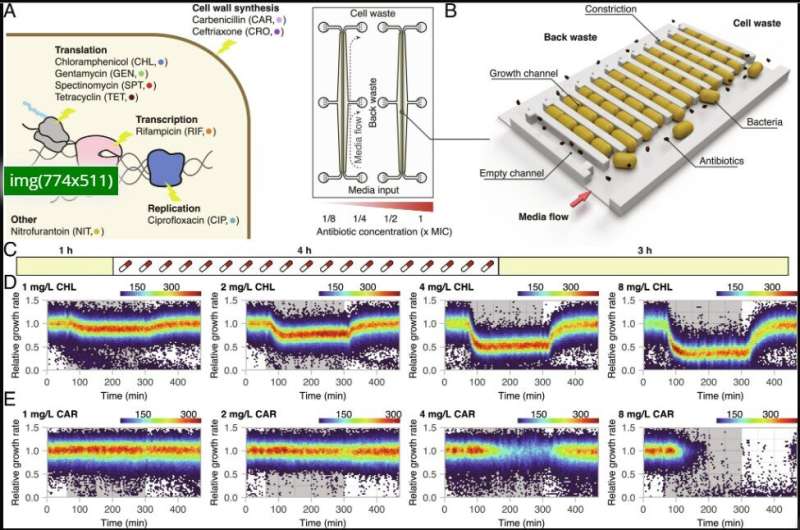
Overview of antibiotic exposure experiment. (A) Antibiotics used in the study. (B) Schematic overview of the microfluidic setup. (C) Timeline of the microfluidic experiments defined by growth in LB (yellow) or LB supplemented with antibiotics (gray). (D and E) Relative growth rates of single cells during the antibiotic exposure experiment. The antibiotic exposure phase is indicated by the gray background. Displayed are representative examples of a bacteriostatic (D) and a bactericidal (E) antibiotic. Credit: Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2216216120
Bacterial perseverance is a new phenomenon that helps explain how bacteria adapt to survive antibiotic treatments. A group of researchers at Uppsala University have studied how individual bacteria react when exposed to different antibiotics. The result underlines the importance of adhering strictly to antibiotic prescriptions.
Fighting bacterial diseases is a perpetual arms race between medical scientists developing new therapeutics and the pathogenic bacteria continuously changing their genetic makeup to survive the drugs.
When antibacterial treatment is initiated, the high concentration of antibiotics kills most bacteria or stops them from growing almost immediately. The patient often feels better after just a few days of treatment, but the rapid recovery can be treacherous. A group of Uppsala researchers has shown that a small fraction of the bacteria often continues to grow, sometimes up to 10 generations.
These bacteria are not resistant. They can continue to divide despite a relatively high concentration of antibiotics due to natural variations in the bacterial strain. But each cell division gives the bacterium a chance to mutate in a way that makes the strain permanently resistant. However, this is a rare event; if you continue your treatment until the last pill, you stand a good chance of clearing the infection.
"This is a new concept that we call antibiotic perseverance," explains Gerrit Brandis, a researcher in the group. "Perseverance describes how a small group of bacteria capable of sustaining growth can accumulate mutations after being exposed to antibiotics. If they are lucky, and the patient unlucky, one of these mutations will allow them to tolerate the antibiotic."
When researchers study bacteria, they typically look at the bacterial population as a whole, that is, they study the collective response of many bacteria. Since perseverant bacteria are very rare, these have so far escaped attention. Johan Elf's lab specializes in studies of individual bacteria and how they react to different stimuli, for example antibiotics. With the help of microfluidic culture chips and AI-based analysis algorithms, they follow the growth of tens of thousands of individual bacteria in one experiment.
"It's a powerful tool," says Elf, who leads the study. "Among other things, it shows the importance of not generalizing, even when it comes to bacteria."
More information: Gerrit Brandis et al, Antibiotic perseverance increases the risk of resistance development, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2216216120
Journal information: Proceedings of the National Academy of Sciences
Provided by Uppsala University