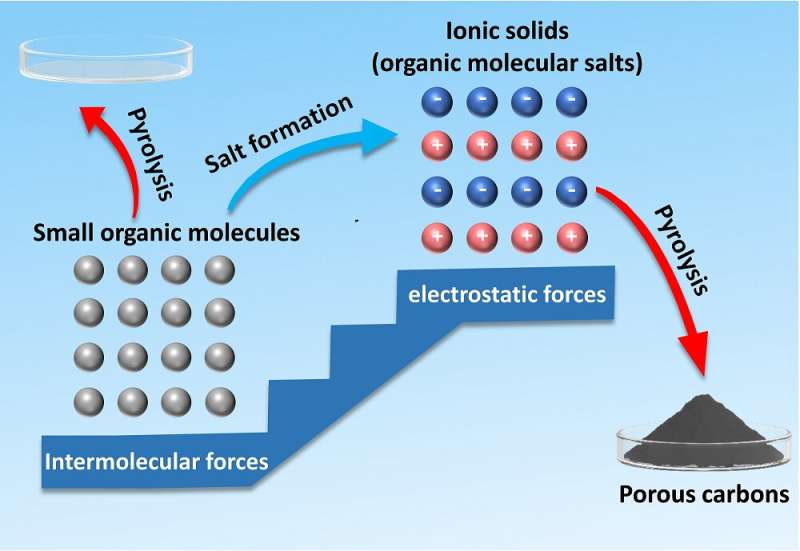
This graphic shows how small organic molecules can be transformed into organic molecular salts, or ionic solids. The organic molecular salts can then be transformed into porous carbons through pyrolysis, which is using high temperatures to activate a transformation. Porous carbons are essential for creating carbon nanostructures, which can be used in a variety of industries. Credit: Nano Research
Small structures made out of carbons are a useful and versatile tool that can be used across industries, including in water and wastewater treatment, gas and oil, and energy storage. In order to create these nanostructures, synthetic and natural polymers have traditionally been used as a starting point to initiate the chemical reaction necessary to create the nanostructured carbons. This is called a precursor.
However, both natural and synthetic polymers have limitations. It is difficult to be as precise with natural polymers because of their complexity and synthetic polymers are difficult and expensive to produce.
Recent research demonstrated an alternative to polymer precursors by using heat to transform small organic molecules into organic metal salts and then into porous carbons. The process of heating the molecules to create a new material is called pyrolysis.
The study was published in Nano Research on October 22.
"The direct purpose of using small molecules as precursors is to simplify carbon preparation by avoiding the polymerization process," said Hai-Wei Liang, a professor and researcher at the University of Science and Technology of China in Hefei.
"More importantly, this concept of using small molecules can greatly expand the structural diversity of precursors for carbon preparation and thus could pave a pathway to study the relationship between carbon material properties and precursor structures."
Previous research into small organic molecules as an alternative to polymer precursors faced limitations due to their synthesis conditions, which created small molecules that were more volatile. This study builds on previous research that showed that using ionic liquids, which is a salt in a liquid state, could resolve some of these limitations.
They took this idea one step further and used organic metal salts, also called ionic solids, instead of ionic liquids, because organic metal salts are both organic and inorganic materials. This combination of organic and inorganic materials helps the organic metal salts form templates for the carbon nanostructures. They also show that a wide range of small organic molecules can be used as precursors, as long as they include acidic groups that can transform into salt.
"The difficulty of using small molecules for carbon preparation mainly comes from their high volatility, which can be easily overcome by the transformation of small molecules into organic metal salts. This is because the original weak intermolecular force holding molecules together is replaced by robust electrostatic force after the salt formation, thus lowering the volatility," said Liang.
Another benefit of using this method is the versatility of the organic metal salts. By altering the components of the organic metal salts, the characteristics of the carbon nanostructures can be controlled at a molecular level.
Looking ahead, researchers will continue to explore the different ways this technique can be used. "Next, we will continue to explore the structural relationship between carbon materials and molecular precursors to establish well-defined rules to guide the rational synthesis of carbon materials at the molecular level. Ultimately, we hope to use the advantage of this method in controlling carbon structures and compositions to achieve the tailor-made synthesis of advanced functional carbon materials for targeted applications," said Liang.
More information: Lei Tong et al, Building the bridge of small organic molecules to porous carbons via ionic solid principle, Nano Research (2022). DOI: 10.1007/s12274-022-4997-8
Journal information: Nano Research
Provided by Tsinghua University Press