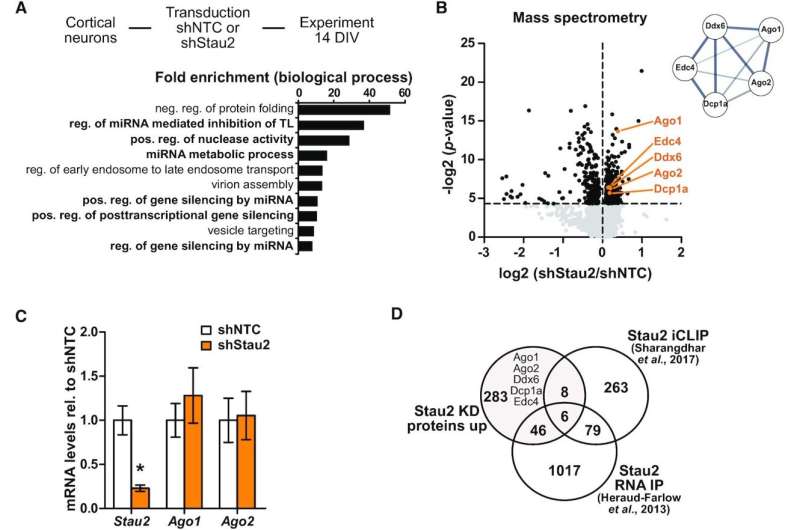
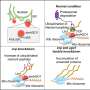
Stau2 depletion leads to upregulation of Ago1/2 and RISC effector proteins. (A) Gene ontology (GO) overrepresentation analysis (PANTHER, biological process) of all significantly upregulated proteins from primary rat cortical neurons deficient for Stau2 from Schieweck et al. (36). (B) Volcano plot displaying protein levels measured by quantitative mass spectrometry and protein network analysis (STRING database) of highlighted significantly upregulated genes (orange; p-value as criteria) from Schieweck et al. (36); unpaired two-tailed Student's t-test. (C) Quantification of Stau2, Ago1 and Ago2 mRNA levels using qRT-PCR from cortical neurons at 14 DIV transduced with shNTC or shStau2 at 10 DIV, normalized to Ppia and shNTC; paired two-tailed Student's t-test. (D) Venn diagram comparing proteins significantly upregulated upon Stau2 KD (36), RNAs enriched in Stau2 RNA-IPs (16), or Stau2 targets identified by iCLIP (12). Error bars are ± SEM from ≥3 independent biological experiments; asterisks represent P-values (*P < 0.05). DIV, days in vitro; NTC, non-targeting control; TL, translation; KD, knock down; iCLIP, individual-nucleotide resolution cross-linking and immunoprecipitation. Credit: Nucleic Acids Research (2022). DOI: 10.1093/nar/gkac487
Neurons constantly adapt to new requirements. This plasticity is the molecular foundation of learning and remembering. At the cellular level, there is a variety of mechanisms for regulating general gene expression.
One of the major players is RNA-binding proteins, which recognize messenger molecules (mRNA). In this way, they regulate where and when proteins can be produced inside the neuron. Jointly with other components, the Staufen2 and Argonaute RNA-binding proteins form RNA granules in the cytoplasm.
A team led by LMU cell biologist Prof. Michael Kiebler has now shown for the first time how Staufen and Argonaute proteins interact with each other.
The authors of the study published in Nucleic Acids Research were able to demonstrate that the two RNA-binding proteins compete with each other in fulfilling their function.
Their results suggest that in this way the two RNA-binding proteins regulate the translation of specific proteins in the dendrite and at the synapse.
The scientists hypothesize that these assembly dynamics of RNA granules make an important functional contribution to synaptic plasticity, particularly in neurons.
More information: Janina Ehses et al, The dsRBP Staufen2 governs RNP assembly of neuronal Argonaute proteins, Nucleic Acids Research (2022). DOI: 10.1093/nar/gkac487
Journal information: Nucleic Acids Research
Provided by Ludwig Maximilian University of Munich