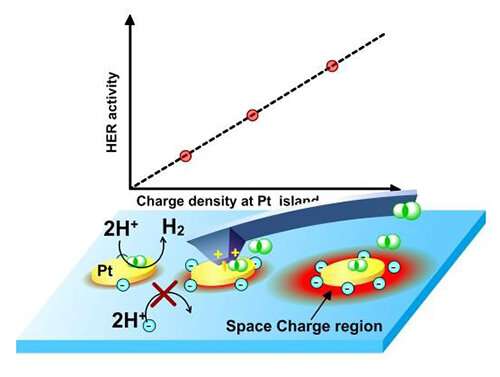
Local HER current on Pt/Ti islands was strictly regulated by the surface charge density in a linear fashion. Credit: NIE Wei and FAN Fengtao
Surface charges play an important role in the catalytic reaction in photoelectrochemistry. However, the spatial heterogeneities of charge transfer sites and catalytic sites at the electrode/electrolyte interface obscures the surface reaction process.
Recently, a research group led by Prof. Li Can and Prof. Fan Fengtao from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) and their collaborators revealed the linear law between surface charge density and reaction current in photoelectrocatalysis.
This study was published in the Journal of Physical Chemistry Letters on Nov. 2.
The researchers quantified the relationship between the local catalytic current of the hydrogen evolution reaction (HER) and the surface charge density using operando spatially resolved photovoltage microscopy on the Pt/Ti array on the p-Si photoelectrode.
They found that the local HER current could be linearly regulated by the charge density at reactive sites by concurrently adjusting the bias potential and the spacings of the Pt/Ti islands.
"Different from the electrocatalytic process in which the applied voltage changes activation free energy, the uniqueness of applied potential on the photoelectrode was found to determine the charge density of photogenerated minorities and hence the reaction current," said Prof. Fan.
More information: Wei Nie et al, Identifying the Role of the Local Charge Density on the Hydrogen Evolution Reaction of the Photoelectrode, The Journal of Physical Chemistry Letters (2021). DOI: 10.1021/acs.jpclett.1c03127
Journal information: Journal of Physical Chemistry Letters
Provided by Chinese Academy of Sciences