Credit: F. Baluška et. al. Phil. Trans.
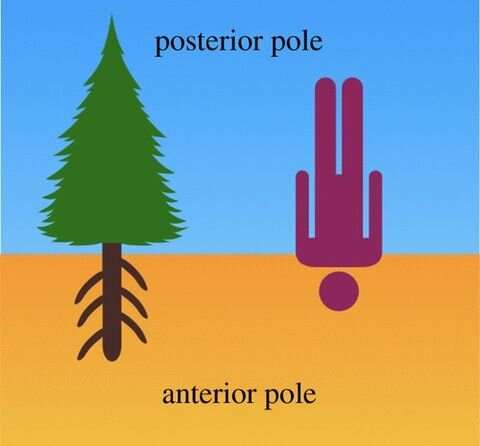
Charles Darwin wrote a book called "The Power of Movement in Plants" with his son Francis in which they first identified the root apex as the central command center of plants. In contrast to our own orientation with respect to Earth's gravitational field, Darwin proposed that the root apices represented the anterior cognitive pole of the plant or tree, while the shoot apices represented the posterior pole. In this view, the root apices are solely responsible for identifying and targeting nutrient-rich and toxin-depleted areas of soil in which to grow, while the shoots generate the sexual apparatus for reproduction.
Another informative brain/plant comparison can be made between the highly polarized cortex and trees. Pyramidal cells extend highly fractalized apical dendrites up into the cortical sheet while puncturing the white matter below with a deeply penetrating and purposively ramified axon. To understand why trees, nervous systems or individual neurons concentrate resources in certain regions within themselves and proliferate uniquely branched elaborations into different external environments, we need to identify the chemical partners and physical persuasions each seeks and responds to.
In the latest special issue of Philosophical Transactions of the Royal Society L. Moroz et al. trace the origins of the most primitive nervous systems to discover how a select few of the thousands of ordinary molecules under cellular control were ultimately knighted into neurotransmitters. While many of the ideas presented in this paper, as well as the larger issue on origins of brains, are still hypothetical, truth is often most readily accepted when transmitted in surprise. Therefore, the unlikely yet inevitable emergence of nervous systems through the simple requirements of extracellular digestion in evolving multicellular forms is an idea that can be readily swallowed once the appropriate chemical links are laid bare.
In this case, the compelling narrative is that peptide or small protein neurotransmitters must have evolved first. The genetic record indicates that secreted proteolytic digestive enzymes and peptide toxins with readily adaptable, three-dimensional structures were the early molecular targets on which natural selection productively operated. Many signaling peptides, mainly originating in the Golgi apparatus, are in turn generated from larger propeptides through successive steps of proteolysis and chemical modification. Cleavages frequently occur at di- or monobasic sites (like lysine-arginine) by prohormone convertases followed by C-terminal α-amidation where a bifunctional peptidylglycine α-amidating monooxygenase (PAM) enzyme converts a C-terminal glycine into an amide.
In a separate article, author Gáspár Jékely provides some additional insights into how peptidergic signaling mechanisms first emerged. He notes that PAM processing predates nervous systems and is present in the green alga Chlamydomonas reinhardtii. It is localized to the cilia of these organisms where it is necessary for their proper formation. Mass-spectrometry screening has revealed that PAM's substrates in Chlamydomonas include chemoattractant peptides that are released on ciliary ectosomes to attract gametes of the minus mating type. The presence of this cell to cell signaling apparatus in green algae reveals the surprisingly deep evolutionary ancestry of the key amidated neuropeptide production line.
Jékely's chemical brain hypothesis postulates that neurotransmitters came before synapses and neurites, as opposed to the other way around. In other words, transmitters make nervous systems. He further suggests that the evolution of circulatory systems and neurohaemal organs released the constraints imposed on peptidergic signaling by diffusion. The so-called hemocoelar circulation within the primary body cavity of invertebrates, coupled with peptide release, ensured the rapid conduction of signals throughout an increasingly large body. While intriguing, it is also true that primitive nervous systems, which predate modern circulatory systems (with oxygenating and immune cells), also distribute nutrients and metabolites, and may have originally evolved for this purpose.
The ultimate cellular nutrient is whole mitochondria. Many cell types, especially immune cells, have a curious penchant for secreting mitochondrial DNA, and often whole mitochondria, within different types of membrane enclosures. They extend special purpose nanotubes (reminiscent of those used in bacterial conjugation exchanges) and tubulin-powered filopodial protrusions to conduct and expel these organelles. Depending on the current state of the donor cell , and whether the acceptor neighbor is friend or foe, they are gifted or assaulted with mitochondria of different health and oxidative states. A more radical, but by no means meretricious hypothesis is that neurons evolved to increase the range and specificity of these kinds of mitochondrial transfers.
In a later article, Detlev Arendt observes that as multicellular animals emerged within a world of host-associated and likely symbiotic microbiota, organisms could have evolved neural phenotypes as immune mediators discriminating self from nonself within their enteric cavities. He notes that there are many similarities between neurons of the ventral nerve tube and our pancreatic islet secretory cells. In addition to similar synaptic machinery for action-potential-stimulated neuropeptide and transmitter release, the combination of transcription factors specifying these cell types is overlapping.
For example, both use the homeodomain factors mnx, nk6, pax6 and Islet, and the onecut transcription factor hnf6 during their early differentiation. These similarities between the vertebrate ventral neural tube and foregut-derived pancreatic islet cells may be evolutionary derivatives of sensory-neurosecretory cells in a digestive mucociliary sole. To this point, the same general transcription factor signature is also shared by select neurons and gut cells in the sea urchin, in the pharyngeal ectoderm derived neurosecretory cell in the cnidarian Nematostella, and in the demosponge Spongilla secretory digestive choanocytes.
The gradual transition from peptide transmitters into singular amino-acid derivatives or other small chemicals centered on just a handful of key players: glutamate, GABA, glycine, ATP, NO and protons. All are relatively cheap and easy to make in abundance in a short period of time. Moroz et. al. explain the universal preservation of these particular molecules in signal transduction operations in terms of an injury/regeneration response. Eating can be a dangerous proposition, particularly if you are a unicellular organism or small colony trying to feed on something comparable to your own size. Feeding often includes an innate immune protection against potential pathogens complete with NO and local deployment of counter-toxins. All the above metabolites are capable of inducing well-coordinated gene expression responses to injury in primitive organisms and in higher plants and animals. A classic example is the role of glutamate in plants where a wound ultimately triggers a long-distance, calcium-based response.
The modern neurotransmitters, including serotonin, dopamine, noradrenaline, adrenaline, octopamine, tyramine, histamine and acetylcholine neurotransmitter pathways, have not been convincingly detected in lower phylogenetic tree. This includes organisms like ctenophores, placozoans, sponges and most of the cnidarians. To date, the most distant homological lineage of any single neuron is probably the meta cerebral cell (MCC). These giant, paired, serotonin-containing interneurons are involved in feeding arousal, and their descendants can be recognized across all Euthyneura (basically snails and slugs). It is a level of molluscan subclasses separated by more than 380 million years of evolution in each direction and therefore of immense importance in understanding early nervous systems.
Much of what we know today about transmitters comes from genetic study of their receptors and their synthesis enzymes. This is a tricky business, because both types of protein are evolutionarily malleable and seemingly change sequence and function at the drop of a hat. For example, the biopterin-dependant tyrosine (TH) and tryptophan (TPH) aromatic amino acid hydroxylases are the rate-limiting enzymes responsible for making catecholamine transmitters and serotonin, respectively. A single mutation in TH (and aspartate to valine at D425V) nearly abolishes enzymatic activity for producing L-DOPA, while increasing the specificity for phenylalanine over tyrosine by 80,000-fold. Similarly, the G-protein coupled receptors that were once optimized to bind and transduce peptide-based signals morphed into detectors of smaller transmitter ligands.
In the octopus, the presence of nicotinic receptors in its suckers was once a source of confusion because they were not sensitive to acetylcholine. It is now appreciated that these receptors are potentially activated by many kinds of chemosensory stimuli and should not be boxed into their original namesake. As the "which came first" story for transmitters and receptors is now rapidly unfolding, the once mysterious origins of nervous systems that puzzled pre-genetic Darwin imagineers now becomes obvious.
More information: Leonid L. Moroz et al. Neural versus alternative integrative systems: molecular insights into origins of neurotransmitters, Philosophical Transactions of the Royal Society B: Biological Sciences (2021). DOI: 10.1098/rstb.2019.0762
Journal information: Philosophical Transactions of the Royal Society
© 2021 Science X Network