Credit: CC0 Public Domain
A research group composed of Professor Takayuki Shibata and his colleagues at Department of Mechanical Engineering, Toyohashi University of Technology, has given greater functionalities to atomic force microscopy (AFM). Our research team has succeeded in minimally invasive surgery to living cells using photocatalytic oxidation controlled in a nanoscale space and visualizing dynamic information on intracellular biomolecules. This proposed technique for controlling and visualizing the process of cell function expression on a high level has significant potential as a strong nanofabrication and nanomeasurement system to solve the mystery of life.
An integrated understanding of life phenomena and the control thereof are absolutely essential for further development of the medical and pharmaceutical fields. The thesis for creating life innovation is to solve the structure and function of biomolecules such as genomes, proteins, and sugar chains and also solve the function of cells, which are the basic unit for life activity. Therefore, we aim to establish a technology for minimally invasive surgery to target living cells at a molecular level (God's hand to manipulate the function of cells) and visualizing changes in the dynamic behavior of intracellular biomolecules and the state of cell membrane protein at a single molecular level (God's eye to see the function of cells), and thus provide an innovative nanofabrication and nanomeasurement platform to solve the mystery of life.
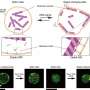
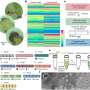
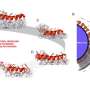
Here, our research team has succeeded in giving two new functions to atomic force microscopy (AFM). The first advancement is to coat the tip apex of an AFM probe with a thin film of titanium oxide (TiO2) known as a photocatalyst. By this method, the photocatalytic reaction is localized in a nanoscale space (100 nm region) in the vicinity of the tip apex to achieve minimally invasive cell membrane perforation. As a result, the probability of cell membrane perforation reaches 100%, and a cell viability of 100% is also successfully achieved, allowing us to verify that minimally invasive surgery can be carried out. The second advancement is to insert the tip apex of an AFM probe coated with silver (Ag) nanoparticles into a living cell. We have thus succeeded in acquiring a sensitive Raman spectrum originating in protein, DNA, lipids, etc. (Tip-Enhanced Raman Spectroscopy, TERS). By this method, a difference in the ratio of biomolecules between a cell's nucleus and cytoplasm was visualized as information inside a cell, and it was found that there is an inverse correlation (a phenomenon that as one increases, the other decreases) between proteins and glycogen (also called animal starch) as temporal changes in biomolecules inside cells.
In order to simultaneously achieve nanofabrication and nanomeasurement functions, we will establish a tip-enhanced Raman spectroscopic (TERS) function by coating the surface of a TiO2-functionalized AFM probe with Ag nanoparticles in the future. This function will be able to visualize the process of degradation reactions of organic substances based on photocatalytic oxidation (changes in molecular structures) during the cell surgery process. We will also aim to achieve a means for measuring a single molecule in a target cell membrane protein using the high molecular recognition ability of an antigen-antibody reaction, and we will aim to establish a technique for selective nanofabrication for a single molecule in the target membrane protein identified by the above means. It is expected that this proposed technique could solve the mechanisms of life functions and be applied to work such as the development of novel medicines.
More information: Takayuki Shibata et al, Photocatalytic Nanofabrication and Intracellular Raman Imaging of Living Cells with Functionalized AFM Probes, Micromachines (2020). DOI: 10.3390/mi11050495
Provided by Toyohashi University of Technology