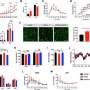
Dissecting the UPR-response with multi-omics approaches, UPR-response is depicted at cellular level red. Credit: © Robert Ahrends
There is a broad range of mechanisms associated with chemoresistance, many of which to date are only poorly understood. The so-called cellular stress response—a set of genetic programs that enable the cells to survive under stressful conditions—plays a key role in the development of numerous diseases and in chemoresistance. A better understanding of the cellular stress response pathways is therefore urgently required to develop new therapeutic concepts to overcome chemoresistance. "In this context, we employed comprehensive analytical approaches to gain deep and molecular insight into the Unfolded Protein Response, a cellular stress reaction induced by unfolded proteins," says Robert Ahrends, group leader at the Department of Analytical Chemistry of the Faculty of Chemistry.
Unfolded proteins cause stress and disease
The Unfolded Protein Response (UPR) contributes to cancer development and progression and plays an important role in diseases such as diabetes and neurodegenerative disorders. For their study of the UPR's molecular biological characteristics, the researchers applied state-of-the-art analytical tools in the context of a multiomics approach, combining large datasets from genetics, proteomics and metabolomics. This allowed them to define the Unfolded Protein Response regulon, a comprehensive list of genes that are activated to promote cell survival under stress.
"Besides the previously known factors, we identified to our surprise numerous genes that have not previously been implicated in stress response pathways," explain the researchers, "and many of them have key functions in cancer development and cellular metabolism."
Changes in 1C metabolism
Changes in cellular metabolism are characteristic of many cancer types and promote a rapid tumor growth, as Nobel Prize winner Otto Warburg demonstrated in the 1930s in his ground-breaking work. In their study, the researchers discovered stress-mediated genetic regulation of enzymes involved in one-carbon (1C) metabolism which relies on the vitamin folate as a cofactor. Concomitant to the metabolic re-wiring, the stressed cells became fully resistant against chemotherapeutic agents, which target this specific metabolic pathway. This includes methotrexate, a drug commonly employed in the treatment of cancer and rheumatic diseases. Detailed biochemical and genetic investigations revealed that resistance is driven by a previously unrecognized mechanism. According to the study authors, its precise molecular characterisation might lead to novel therapeutic concepts aimed at overcoming chemoresistance in cancer therapy.
More information: Stefan Reich et al, A multi-omics analysis reveals the unfolded protein response regulon and stress-induced resistance to folate-based antimetabolites, Nature Communications (2020). DOI: 10.1038/s41467-020-16747-y
Journal information: Nature Communications
Provided by University of Vienna