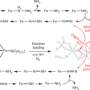
Utah State University biochemist Karamatullah Danyal uses a quench flow instrument in a glove box while studying the steps of nitrogen fixation. Danyal is part of a USU team that published two papers in the Sept. 23 online Early Edition of Proceedings of the National Academy of Sciences. Photo by M. Muffoletto.
Utah State University scientists have published two papers in a high profile academic journal this week that unlock mysteries of a chemical process upon which all life on earth depends.
In each paper, the researchers, under the leadership of USU biochemistry professor Lance Seefeldt, describe newly discovered insights about nitrogen fixation, a process that converts life-sustaining nitrogen into a form that humans, animals and plants can access.
"It's an incredible irony," says Seefeldt, professor in USU's Department of Chemistry and Biochemistry. "Nitrogen, in the form of dinitrogen, makes up about 80 percent of the air we breathe. We need it to survive and we're swimming in a sea of it, yet we can't get to it."
Humans and animals consume nitrogen in the form of protein from food. Plants obtain nitrogen from the soil.
Sounds simple, but it isn't. Nitrogen fixation is a complex and energy-intensive process. In papers published in the Sept. 23, 2013, online Early Edition of Proceedings of the National Academy of Sciences, Seefeldt and USU colleagues Edwin Antony, Zhi-Yong Yang, Simon Duval, Karamatullah Danyal, Sudipta Shaw, Anna Lytle, Nimesh Khadka, along with Dennis Dean of Virginia Tech and Dmitriy Lukoyanov and Brian Hoffman of Northwestern University report research breakthroughs. Their research is supported by the National Institutes of Health.
"Dinitrogen consists of two nitrogen atoms joined by one of the strongest triple bonds known in chemistry," says Danyal, a doctoral candidate in Seefeldt's lab. "Breaking these bonds requires tremendous energy, which is released during a cycle of events associated with the transfer of metallic electrons and a chemical reaction called ATP hydrolysis."
USU researchers, as well as others in the science community, have studied steps of this cycle for decades. Yet the nature of the coupling between the electron transfer and ATP hydrolysis and the order in which each occurs has eluded them. Until now.
"With key experiments, we've demonstrated the electron transfer precedes ATP hydrolysis," says Antony, assistant professor in USU's Department of Chemistry and Biochemistry.
In the second paper, the research team describes the mechanism for hydrogen formation during nitrogen fixation.
"This is a profound insight," says Yang, a doctoral candidate in Seefeldt's lab. "We previously predicted how this could happen, but now we've demonstrated how it happens."
The researchers' discoveries about nitrogen fixation could have energy-saving implications for the world's food supply.
"Two known processes break nitrogen bonds and allow conversion," Seefeldt says. "One is a natural, bacterial process. The other is the man-made Häber-Bosch process. The world's food supply currently depends equally on each of these."
The century-old Häber-Bosch process, used to make agricultural fertilizers, is energy-intensive and depends heavily on fossil fuels, Seefeldt says, so interest is high in harnessing and making more use of the cleaner, natural process.
"By finally unlocking the conversion process, we can look to Mother Nature for answers," he says.
More information:
On reversible H2 loss upon N2 binding to FeMo-cofactor of nitrogenase, www.pnas.org/cgi/doi/10.1073/pnas.1315852110
Electron transfer precedes ATP hydrolysis during nitrogenase catalysis, www.pnas.org/cgi/doi/10.1073/pnas.1311218110
Journal information: Proceedings of the National Academy of Sciences