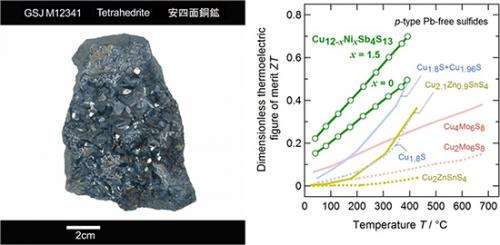
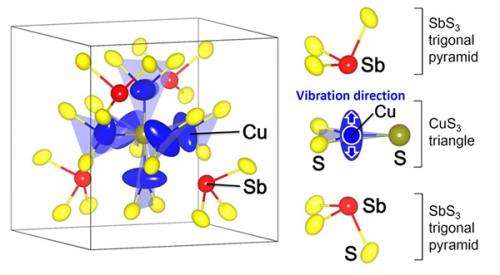
Figure 1 : Natural tetrahedrite (Cu, Fe, Ag, Zn)12Sb4S13 (sample owned by Geological Museum, AIST)(left) and dimensionless thermoelectric figure of merit, ZT, for the tetrahedrite Cu12-xNixSb4S13 and various p-type Pb-free sulfides(right)
Researchers with the National Institute of Advanced Industrial Science and Technology have confirmed that a naturally occurring mineral, tetrahedrite, which mainly consists of non-toxic and earth-abundant elements, copper (Cu) and sulfur (S), exhibits high thermoelectric performance at approximately 400 ℃. They also clarified that the high performance is attributed to its extremely low lattice thermal conductivity caused by the complex crystal structure of tetrahedrite and the vibration of Cu atoms with anomalously large amplitude. These efforts will significantly contribute to realizing environmentally friendly thermoelectric power generation using materials composed of non-toxic and earth abundant elements.
Thermoelectric power generation is a technology that can convert thermal energy (in the form of a temperature difference) into electrical energy using solid devices. With the recent growing interest in energy problems, thermoelectric power generation has been attracting attention as a possible means of effectively utilizing the huge amount of unused waste heat. In particular, it is desired to recover and use intermediate-temperature (300-500 ℃) waste heat from automobiles and plants. Promising thermoelectric materials for the temperature range consists of toxic elements such as lead (Pb) and this fact is an obstacle to practical use.
In 2011, the research group of JAIST started searching for thermoelectric materials containing Cu and S, which are non-toxic and earth-abundant elements. About a year ago, the group artificially synthesized a material with a composition almost identical to that of tetrahedrite, a naturally occurring sulfide mineral, and reported its relatively high thermoelectric performance at room temperature in an international journal published by the Japan Society of Applied Physics. The scientists of JAIST, in cooperation with those from AIST with remarkable achievements in the research on thermoelectric sulfides, continued the research and found that nickel(Ni)-substituted Cu12Sb4S13, Cu12-xNixSb4S13, exhibits a high dimensionless thermoelectric figure of merit of ZT=0.7 (equivalent to conversion efficiency of 7 %) at approximately 400 ℃. This value is the highest among p-type Pb-free sulfides so far. Tetrahedrite is now attracting worldwide attention as a promising thermoelectric material. At the end of 2012, a research group from Michigan State University (USA) also reported high thermoelectric performance in tetrahedrite Cu12-xMxSb4S13 (M=Zn:zinc or Fe:iron).
Figure 2 : Part of crystal structure of tetrahedrite Cu atoms vibrate with large amplitude in a direction perpendicular to the CuS3 triangle plane and toward the Sb atom.
The high performance of tetrahedrite is caused by its extremely low lattice thermal conductivity, which is approximately half that of silica glass. A powder X-ray diffraction experiment was carried out using synchrotron radiation at SPring-8, which has been used to analyze the structural properties of various thermoelectric materials, to investigate the crystal structure of the Ni-substituted tetrahedrite and the vibrational properties of its atoms in detail. The results indicate that the Cu atom on the CuS3 triangular plane vibrates with low frequency and large amplitude in the direction perpendicular to the triangle plane. From this finding, the vibration of Cu atoms with anomalously large amplitude is considered to inhibit heat transfer through the hard Cu-Ni-Sb-S network, resulting in the low thermal conductivity.
In this study, the sulfide mineral tetrahedrite, a well-known mineral resource, was found to be a promising thermoelectric material. Clarification of the characteristics of the crystal structure for the mineral resulting in its low thermal conductivity will lead to the development of high-performance and environmentally friendly thermoelectric sulfide minerals.
The research results were published online in Journal of Applied Physics on January 28, 2013.
Journal information: Journal of Applied Physics
Provided by Advanced Industrial Science and Technology