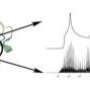
Experimental research and theoretical calculations show that water resides inside the calix[4]arene cavity, not on the lip as earlier studies indicated. Image: Courtesy of JC Werhahn, Dept. of Physics, Technical Univ. of Munich at Garching, Germany
(PhysOrg.com) -- By combining theoretical and experimental expertise in the United States, Japan, and France, a team of scientists determined that a water molecule (guest) is cradled inside a functional molecule (host), turning it into the world's smallest cup of water.
The location of a guest makes a difference in the host's behavior and function. The study also cautioned about some commonly used low-scaling methods that are widely used to model those systems in aqueous environments.
This research was performed by scientists from Hiroshima University, the Universite Paris-Sud 11, and Pacific Northwest National Laboratory. This work was featured in the Benoît Soep Festschrift special issue of the Journal of Physical Chemistry A.
Scientists in biology and other fields need to know the relative strength of the interaction of water with different functional groups that are part of a biomolecule. This study provided information about why water chooses different locations to bind to the host, based on two competitive interactions: one interaction is hydrogen bonding outside the host and the other the water p-interaction with the aromatic rings inside the host. These interactions are prototypical in the study of how biological molecules, such as proteins, function in aqueous solutions. This research is important in modeling the interaction of water with biological systems, such as proteins, and ensuring that the theoretical models can accurately predict the relative strength of those two competitive interactions.
To begin, the researchers selected calix[4]arene, a functional molecule that often stands in for proteins. It was chosen because researchers can easily study different bonding scenarios and their influence on the molecule's function and selectivity in aqueous environments. Of particular interest was the competition between the above two types of interactions.
The researchers in Japan formed the calix[4]arene-water complexes. They investigated their structure using experimental techniques such as mass-selected resonant two-photon ionization spectroscopy, infrared-ultraviolet double resonance spectroscopy, and infrared photodissociation spectroscopy. The researchers from Paris did the preliminary theoretical calculations, specifically density functional theory calculations. The U.S. scientists did additional, more complex electronic structure calculations. These calculations were done at the Department of Energy's EMSL.
The experimental and theoretical calculations both converged to the conclusion that water rests inside the calix[4]arene. This research provides two major benefits to the scientific community. First, using the model calix[4]arene host, it allows for the quantitative description of guest/host interactions in functional materials. Second, it provides an important benchmark about the accuracy of electronic structure methods that are typically used to model the function of biological systems in aqueous environments. It was, for instance, found that some of the most commonly used density functional theory approaches rendered an incorrect picture about the strength of the relative interactions when compared to more accurate electronic structure methods.
More information: Hontama N, Y Inokuchi, T Ebata, C Dedonder-Lardeau, C Jouvet, and SS Xantheas. 2010. "The Structure of the Calix[4]arene-(H2O) Cluster: The World's Smallest Cup of Water." Journal of Physical Chemistry A 114(9):2967-2972. DOI:10.1021/jp902967q
Provided by Pacific Northwest National Laboratory
![Experimental research and theoretical calculations show that water resides inside the calix[4]arene cavity, not on the lip as earlier studies indicated.
Image: Courtesy of JC Werhahn, Dept. of Physics, Technical Univ. of Munich at Garching, Germany The World's Smallest Cup of Water: Team Shows Location of Water Relative to Prototypical Protein](https://scx1.b-cdn.net/csz/news/800a/theworldssma.jpg)