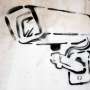
Crystals of sodium cobaltate grown at the Clarendon Laboratory. Credit: Stuart Bebb, Oxford University’s Department of Physics.
Longer-lasting laptop and mobile phone batteries could be a step closer thanks to research by scientists at the University of Oxford.
Researchers from Oxford’s Department of Physics are part of an international team investigating sodium cobaltate: a material similar in structure to the lithium cobaltate used in rechargeable batteries for many electronic devices.
In a recent Nature paper the team describe the patterning of ions in sodium cobaltate– observations that are helping to unlock the secrets of how ions move around inside the electrodes of lithium-based batteries. Their findings could lead to improvements in battery design.
Vital to their success were the very high quality single crystals of sodium cobaltate grown by Dr D Prabhakaran in the Clarendon Laboratory. ‘The hardest part was learning how to control the concentration of sodium in the crystals,’ Dr Prabhakaran said. ‘I achieved this by using a special type of floating-zone furnace that allows the growth of crystals in a high-pressure atmosphere and reduces the evaporation of sodium.’ These samples were then bombarded with neutrons to reveal how the sodium ions were arranged.
Professor Andrew Boothroyd, a member of the Oxford team, said: ‘If you want to engineer new materials with improved properties you need to know how they work. Knowing the forces which drive the sodium ion ordering we can start to understand how the ions move through the structure and explain many of the observed electrical and magnetic properties of this fascinating material – such as a superconducting phase that only develops after you immerse it in water.’
Source: Oxford University