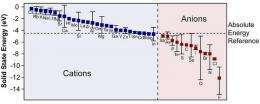
A new "solid state energy scale" developed by researchers at Oregon State University provides a new way to understand electronegativity, a fundamental but very important concept in chemistry. (Graphic courtesy of Oregon State University)
Researchers at Oregon State University have created a new, unifying method to describe a basic chemical concept called "electronegativity," first described almost 80 years ago by OSU alumnus Linus Pauling and part of the work that led to his receiving the Nobel Prize.
The new system offers simplicity of understanding that should rewrite high school and college chemistry textbooks around the world, even as it opens important new avenues in materials and chemical research, with possible applications in everything from solar energy to solid state batteries.
The findings were just published in the Journal of the American Chemical Society, in work supported by the National Science Foundation and the U.S. Department of Energy.
"This is a quantum leap forward in understanding basic tendencies in chemical bond formation," said John Wager, a professor of electrical engineering at OSU. "We can now take a concept that college students struggle with and I could explain it to a kindergarten class.
"Even advanced scientists will gain new insights and understanding into the chemical processes they study," Wager said. "Using this system, I could look at various materials being considered for use in new solar energy cells and determine quickly that this one might work, that one doesn't stand a chance."
Electronegativity, as defined by Pauling, is "the power of an atom in a molecule to attract electrons to itself." This concept is useful for explaining why some atoms tend to attract electrons, others share them and some give them away. In the 1930s, Pauling was the first to devise a method for numerically estimating the electronegativity of an atom. Other researchers later developed different approaches.
The new system developed at OSU – the first of its type since the early 1990s - is called an atomic "solid state energy scale." It characterizes electronegativity as the solid state energy of elements in a compound, and shows that electrons simply move from a higher energy to a lower energy.
"This is a remarkably intuitive approach to understanding electronegativity, and yet it's based on data that are absolute, not arbitrary," said Douglas Keszler, an OSU professor of chemistry, co-author on the study and an international expert in materials science research.
"This is already one of the best instruments in my tool box for predicting the properties of new materials and understanding inorganic reactions," Keszler said. "It's not only more accurate and comprehensive, it just offers a simplicity of understanding that is very important."
The electronegativity scale developed by Pauling is among the most widely known of his contributions in studies on the nature of the chemical bond, the work for which he received a Nobel Prize in chemistry.
According to Ram Ravichandran, an electrical engineering student at OSU and co-author of the study, the new approach is based on the study of how the "band gap," a fundamental property of materials, varies for a variety of compounds. This helps to derive an absolute energy reference and a new solid state energy scale, providing a surprisingly simple way to visualize the way materials will interact.
The system could aid research in new semiconductor devices, catalysts, solar cells, light emitting materials and many other uses.
Provided by Oregon State University