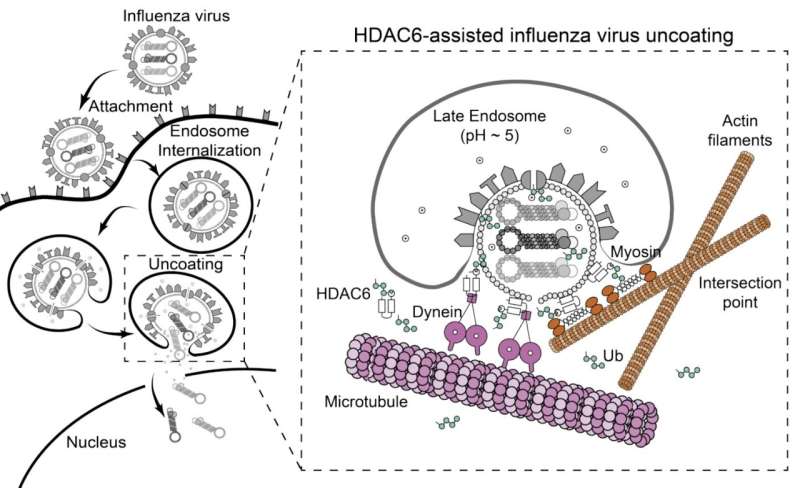
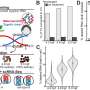
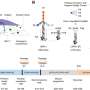
Model of HDAC6-assisted influenza virus uncoating. Credit: Cell Reports (2023). DOI: 10.1016/j.celrep.2023.113558
Influenza and other viruses pack their genetic material into a protein shell, which must be disassembled for the viruses to efficiently replicate. But how viruses "uncoat" their genes remains largely unknown. Now, Friedrich Miescher Institute researchers have identified crucial features of this uncoating process—work that may inform the development of new antiviral treatments.
Seasonal flu, caused by the influenza A virus, is an acute respiratory infection that can lead to severe illness or death, particularly among the elderly and people with serious medical conditions. Like other viruses, influenza A is a master hijacker that takes control of the machinery of an infected cell to produce new viral particles. The virus's genetic material is enclosed within a protective protein shell, which must be taken to pieces through a process called uncoating.
Scientists have known that during uncoating, chains of a protein called ubiquitin—which are attached to the surface of the influenza A virus—interact with a cellular enzyme known as HDAC6. HDAC6 in turn binds to components of the cell's skeleton and to motor proteins, pulling the virus's protective shell into pieces. However, how viral uncoating works remains unclear.
To investigate this process, Longlong Wang, a postdoctoral fellow in the Matthias group, and his collaborators at the ETH Zurich and the University of Geneva combined lab-based experiments with mathematical modeling. The researchers found that ubiquitin acts as a bridge between HDAC6 and myosin, a key cytoskeletal protein.
Actin, another cytoskeletal protein that binds myosin, is also crucial for the opening of the virus's outer shell. The mathematical modeling also showed that these cytoskeletal proteins can indeed generate the forces required for pulling the virus's outer shell into pieces.
These findings, published last month in Cell Reports, suggest that preventing the binding of HDAC6 to ubiquitin can help fight infections with influenza A and other viruses. "The interaction between HDAC6 and ubiquitin could be a good therapeutic target," Wang says.
Next, the researchers plan to investigate ubiquitin's role in the life cycle of the influenza A virus and how the protein triggers the host's immune response.
More information: Alina Artcibasova et al, A quantitative model for virus uncoating predicts influenza A infectivity, Cell Reports (2023). DOI: 10.1016/j.celrep.2023.113558
Journal information: Cell Reports