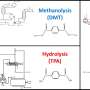
Gem-dimethyl incorporation strategy for the modification of tylophorine. Credit: Acta Materia Medica (2023). DOI: 10.15212/AMM-2023-0032
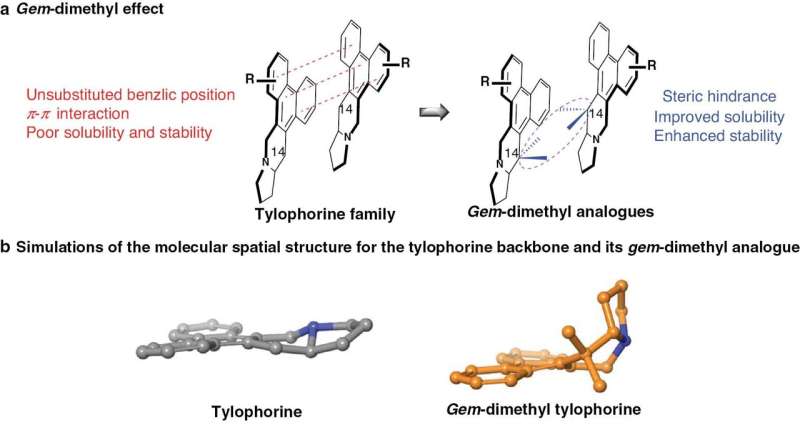
Tylophorine has diverse biological activities; however, the stability, solubility, and central nervous system toxicity have severely limited use of tylophorine. The gem-dimethyl group is an organic chemistry functional group that consists of two methyl groups bonded to the same carbon atom. This feature has gained significant attention in medicinal chemistry due to its unique properties and potential applications in drug design.
The authors of the article published in Acta Materia Medica applied a new photoredox methodology to tylophorine modification, resulting in a series of gem-dimethyl tylophorine analogs. Among the analogs, compound 4b demonstrated promising activity against a wide range of tumor cell lines and exhibited significantly improved drug-like properties, including enhanced solubility and stability.
Compound 4b showed an exceptional inhibitory effect (7.8 nM) against a C481S mutation-induced ibrutinib-resistant non-Hodgkin's lymphoma cell line, as well as primary tumor cell lines obtained from patients. Importantly, compound 4b exhibited significantly reduced anti-proliferative activity against the normal cell line tested, indicating the potential for an enhanced therapeutic window for compound 4b.
Based on these early-stage data, this study provides a solid foundation for the development of new therapeutic agents for potential drug-resistant cancer treatment in the near future.
More information: Chao Zhang et al, Photoredox-catalyzed reaction as a powerful tool for rapid natural product Gem-dimethylation modification: discovery of potent anti-cancer agents with improved druggability, Acta Materia Medica (2023). DOI: 10.15212/AMM-2023-0032
Provided by Compuscript Ltd