Credit: Unsplash/CC0 Public Domain
Agricultural and forestry waste biomass is a renewable lignocellulosic resource. The cellulosome-producing strain Clostridium thermocellum (C. thermocellum), an efficient cellulose-degrading bacterium, is a promising component for lignocellulose biorefinery.
Recently, researchers from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences (CAS) revealed the significant role of β-glucosidase (BglA) in the metabolism of cellobiose and laminaribiose in C. thermocellum. Their results were published in the International Journal of Biological Macromolecules.
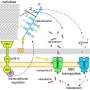
Previous studies suggested that C. thermocellum primarily converts oligosaccharides into glucose and glucose-1-phosphate through the phosphorolytic pathway, which then enters the glycolytic pathway for assimilation. The BglA-based hydrolytic pathway remains not fully elucidated.
Based on enzymatic activity analysis, gene knockout, and transcriptomic analyses, the researchers determined the key role of BglA in the metabolism of both β-1,4-glycosidic bond containing cellodextrins and β-1,3-glycosidic bond containing laminaribiose. With laminaribiose as substrate, the activity of BglA was 24 times higher than that on cellobiose.
Furthermore, they determined the structural basis for specific hydrolysis by crystal structure determination and molecular docking analysis. They also revealed the specific cross-regulation of the hydrolytic pathway mediated by BglA and the phosphorolytic pathway mediated by phosphorolytic enzymes in C. thermocellum.
More information: Yan Xiao et al, Key roles of β-glucosidase BglA for the catabolism of both laminaribiose and cellobiose in the lignocellulolytic bacterium Clostridium thermocellum, International Journal of Biological Macromolecules (2023). DOI: 10.1016/j.ijbiomac.2023.126226
Provided by Chinese Academy of Sciences