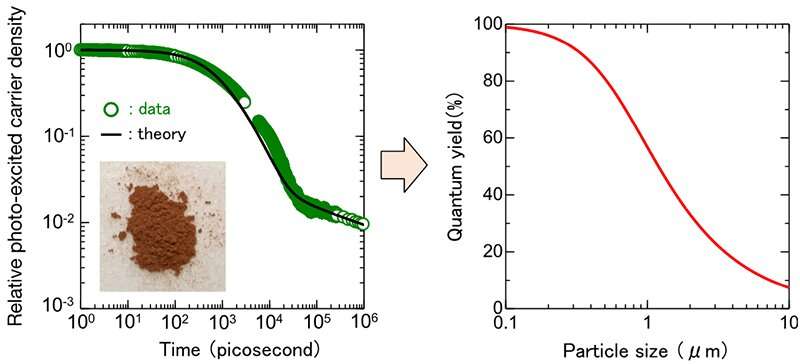
(Left) Time evolution of the photo-excited carrier concentration of the oxysulfide photocatalyst Y2Ti2O5S2 with respect to that at 1 picosecond after light irradiation. The photograph shows the powdered Y2Ti2O5S2 used for this measurement. (Right) Performance prediction with the size of a photocatalyst particle. Credit: Advanced Industrial Science and Technology
In 2019, Shinshu University Special Contract Professor Domen Kazunari and colleagues developed the powdered oxysulfide photocatalyst Y2Ti2O5S2 that absorbs sunlight of wavelengths below 650 nm and splits water into hydrogen and oxygen.
This photocatalyst continuously splits water into hydrogen and oxygen at a volume ratio of 2:1 over a period of 20 hours, and theoretically can be expected to achieve a conversion efficiency of more than 10%. However, the current conversion efficiency is less than 1% and further improvement of photocatalyst is needed, but the guidelines were not clear.
In collaboration with research partners, AIST researchers have now clarified the conditions needed for an oxysulfide photocatalyst Y2Ti2O5S2, which splits water into hydrogen and oxygen under visible light, to achieve a conversion efficiency from solar energy to reaction energy (hereafter, "conversion efficiency") over 10% for practical use.
In this research, by using transient absorption spectroscopy, the photo-excited carrier concentration of Y2Ti2O5S2 was recorded with time over the range of six orders of magnitude from 1 picosecond to 1 microsecond, and physical properties such as the lifetime of photo-excited carrier (hereafter, "carrier lifetime") in powder form were obtained by analyzing the recorded data.
Then, simulations were performed using the physical properties to obtain the relationship between the conversion efficiency and the powder particle size. It was found that the conversion efficiency could exceed 10% by reducing the particle size below 1 micrometer. Furthermore, simulation analysis assuming the doping effect to extend the carrier lifetime suggested the conversion efficiency larger than 10% by reducing the electron concentration to 1/100th of the current level.
The results of this research provide a quantitative guideline for further enhancing the conversion efficiency of the oxysulfide photocatalysts and development of new materials that more efficiently generate hydrogen from water.
Provided by Advanced Industrial Science and Technology