Credit: POSTECH
Bacteria, fungi, and microalgae—living things too small to be seen with the naked eye—are microorganisms that are commonly used for chemical production via their fermentation. The development of Mycoplasma mycoides in 2010, an artificial microorganism, has highlighted the technology that is utilized to develop industrial microorganisms such as Escherichia coli and yeast as "cell factories" for producing petroleum-substituting chemicals and pharmaceuticals.
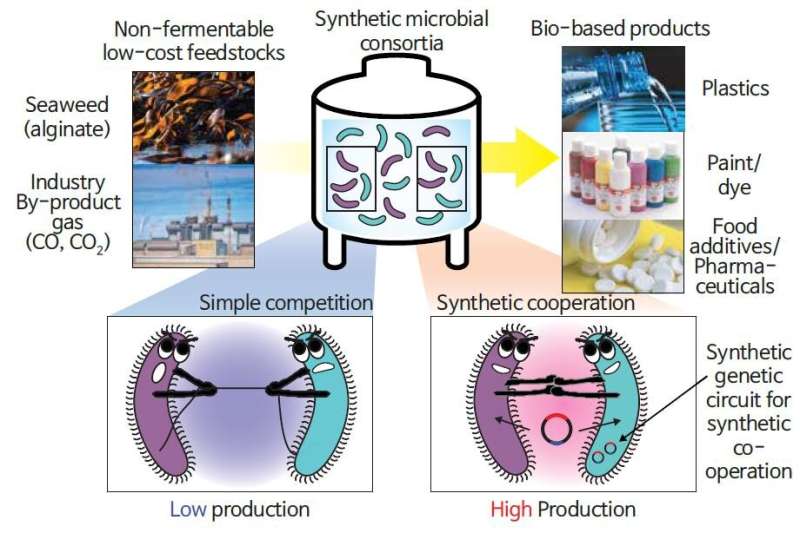
However, bioprocesses are often limited to pure cultures of a single strain. Although multiple strains can be grown together for diverse processes, these strains will inevitably compete—just as humans do—for limited resources, lowering the overall productivity.
A joint research team of Professor Gyoo Yeol Jung, Dr. Chae Won Kang, and Dr. Hyun Gyu Lim (Department of Chemical Engineering) at POSTECH in collaboration with Professor Jaeyoung Sung and Ph.D. candidate Jaehyuk Won (Department of Chemistry) from Chung-Ang University has developed a new bioprocess by introducing a genetic circuit, named "population guider," into a co-culturing consortium, which induces cooperation among microorganisms to improve productivity. The findings from this study were published in the latest issue of Nature Communications.
Most current microbial processes for chemical production use pure cultures of a single strain to ensure stable production. However, engineering a single strain for performing multiple functions at a time is challenging. An alternative method would be the co-cultivation of two or more strains with different capabilities, but stable production cannot be guaranteed because of a competition toward their individual growth.
To overcome these issues, the research team used a genetic circuit, an important technology in synthetic biology, as a "guide" for microorganisms. The researchers designed a consortium of Vibrio sp. dhg, which utilizes alginate typically found in kelp and other seaweed, and Escherichia coli strain, which produces 3-hydroxypropionic acid (3-HP) that are used as raw material for paints, dyes, fabrics, diapers, etc., for the direct conversion of alginate to 3-HP.
They introduced a "population guider" genetic circuit into the E. coli strain, which degrades ampicillin only when 3-HP is produced. In the presence of ampicillin as a selection pressure and microorganism cooperation facilitator, the consortium was successfully acclimated, increasing 3-HP production 4.3-fold over the output of a simple co-culturing consortium during fermentation.
The lead investigator Professor Jung explained, "Our findings are the first to suggest that an artificial genetic circuit in multiple microbial strains to prevent their competition from lowering productivity in chemical production. This new technology is acclaimed as an innovation that can secure both higher productivity and flexibility in microbial bioprocesses.'"
More information: Chae Won Kang et al, Circuit-guided population acclimation of a synthetic microbial consortium for improved biochemical production, Nature Communications (2022). DOI: 10.1038/s41467-022-34190-z
Journal information: Nature Communications
Provided by Pohang University of Science & Technology