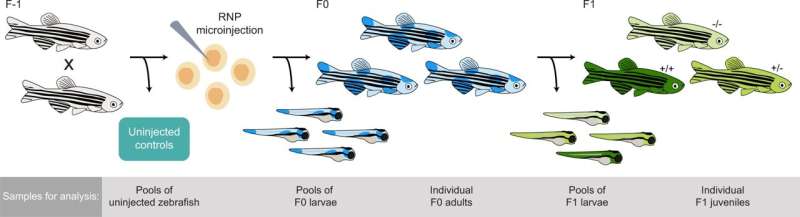
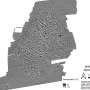
Overview of CRISPR-Cas9 genome editing in zebrafish. Genome editing was performed in fertilized eggs by microinjection of ribonucleoprotein (RNP) at the single-cell stage. The genome editing experiment results in mosaic heterozygous mutants, mosaic homozygous mutants, or unaffected homozygotes (together referred to as founders). A number of F0 embryos were not injected and used as controls. F1 generation zebrafish were generated by in-crossing randomly selected pairs of adult founders. The offspring of these crossings have stable genotypes with 0 (−/−), 1 (+/−) or 2 (+/+) mutated alleles. Samples were collected for analysis at different stages of the experiment as described in the gray box. Credit: DOI: 10.1038/s41467-022-28244-5
CRISPR-Cas9, the "genetic scissors," creates new potential for curing diseases, but treatments must be reliable. In a new study, researchers have discovered that the method can give rise to unforeseen changes in DNA that can be inherited by the next generation. These scientists therefore urge caution and meticulous validation before using CRISPR-Cas9 for medical purposes.
CRISPR-Cas9 is an effective tool for genome modification in microorganisms, as well as animals and plants. In health care, the method creates scope for curing numerous genetic diseases, provided the DNA is modified correctly and undergoes no unexpected changes. To date, such unwanted mutations have been studied in cells, but knowledge of the consequences in living organisms remains limited.
"In this project, we studied the effects of CRISPR-Cas9 in zebrafish, a small aquarium fish. Since DNA molecules and their mechanisms are similar in all animals, we think the results should be similar in humans, for example," says Adam Ameur, associate professor at Uppsala University and the Science for Life Laboratory (SciLifeLab).
When they studied the genome of more than 1,000 zebrafish from two generations, the researchers found unexpected mutations of various types. In some cases, DNA fragments that were larger than anticipated underwent changes, while in other cases mutations occurred in the wrong location in the genome. Unforeseen mutations were found in first-generation zebrafish, but also in their offspring.
"Knowing these unexpected mutations are heritable is important, since they can have long-term consequences for future generations. But that can happen only if you change the genome of embryos or germ cells," says Ida Höijer, Ph.D. of Uppsala University and SciLifeLab.
In healthcare, methods tailored to correct genes in a particular tissue or cell type are now being developed. Although such treatments pose no risk to future generations, caution is advisable.
"CRISPR-Cas9 can be an amazingly valuable tool in health care. But we need to minimize the risk of unwanted effects, and we can do this by carefully validating the modified cells with the latest DNA sequencing technologies," Ameur says.
The research is published in Nature Communications.
The study was led by researchers at Uppsala University and SciLifeLab's National Genomics Infrastructure (NGI).
More information: Ida Höijer et al, CRISPR-Cas9 induces large structural variants at on-target and off-target sites in vivo that segregate across generations, Nature Communications (2022). DOI: 10.1038/s41467-022-28244-5
Journal information: Nature Communications
Provided by Uppsala University