Credit: DOI: 10.1021/acs.jnatprod.1c00461
A research group from the South China Sea Institute of Oceanology of the Chinese Academy of Sciences reported the structure diversification of bioactive atypical angucyclines by inactivating redox enzyme-encoding genes in fluostatin biosynthesis.
This study was published in Journal of Natural Products on August 12.
Atypical angucyclines are a class of important natural products with antibacterial, antitumor and enzyme inhibition activities. Biosynthetic redox enzymes often play significant roles in bringing structure richness of natural products.
In the biosynthetic gene clusters (BGCs) of atypical angucyclines, a number of redox enzymes have been found, but their biosynthetic functions are still unclear.
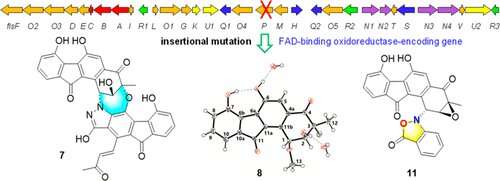
In this study, the researchers carried out in vivo gene disruption of a redox enzyme-encoding gene flsP in the fluostatin BGC, and reported the structure diversification of fluostatin-related atypical angucyclines. Bioinformatics analysis showed that the flsP encoding oxidoreductase was likely involved in the modification of the highly oxygenated A-ring in fluostatins.
A subsequent insertional deletion of flsP in Micromonospora rosaria SCSIO N160, the fluostatin producer, led to the discovery of a series of atypical angucyclines derivatives.
In their previous study published in The Journal of Organic Chemistry, four new angucyclinone derivatives had been discovered by inactivation of the flavoenzyme-encoding gene flsO1. Notably, FlsO1 was revealed to be a benzofluorene hydroxylase that was functionally equivalent to its homologous enzyme AlpK in kinamycin biosynthesis.
More information: Chunshuai Huang et al, Discovery of an Unexpected 1,4-Oxazepine-Linked seco-Fluostatin Heterodimer by Inactivation of the Oxidoreductase-Encoding Gene flsP, Journal of Natural Products (2021). DOI: 10.1021/acs.jnatprod.1c00461
Journal information: Journal of Natural Products
Provided by Chinese Academy of Sciences