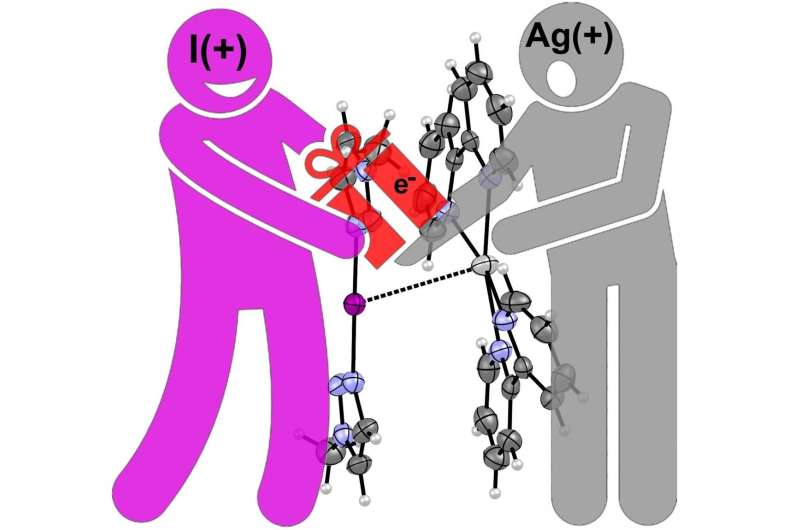
How it happens: Positive iodine gives an "electron gift" to positive silver. Credit: Antonio Frontera and Kari Rissanen
An international research team led by Professor Kari Rissanen of the University of Jyvaskyla (Finland) and Professor Antonio Frontera of the University of Balearic Islands (Spain) has demonstrated that positively charged iodine (termed iodonium) is able to favorably interact with a silver cation (Ag+), overcoming the strong electrostatic repulsion. The research was published online in Chem journal February 8, 2021.
It is well known and intuitive that iodide (I-) has a strong affinity for Ag+. For instance, AgI is one of the most insoluble inorganic salts due to the strength of their attractive electrostatic force. In fact, it is used to generate artificial rain (cloud seeding) because the crystalline structure of AgI is similar to that of ice (ideal nucleation agent).
However, the strong and counter-intuitive affinity of iodonium (I+) for Ag+ was not known until now. An international research group have succeeded in preparing and characterizing a supramolecular complex where I+ and Ag+ are in close contact, thus overcoming the intrinsic electrostatic repulsion of their positive charges. Remarkably, the I+···Ag+ interaction was demonstrated both in the solid state and in solution.
"The explanation provided is that the iodine atom, even as a positively charged cation, is able to generously donate electrons from its free electron lone pairs to the Ag+. Therefore, no matter the electronic nature of iodine, rich or poor, it seems its generosity toward the Ag+ is boundless," says Professor Kari Rissanen from University of Jyvaskyla.
More information: Shilin Yu et al, A "nucleophilic" iodine in a halogen-bonded iodonium complex manifests an unprecedented I+···Ag+ interaction, Chem (2021). DOI: 10.1016/j.chempr.2021.01.003
Journal information: Chem
Provided by University of Jyväskylä