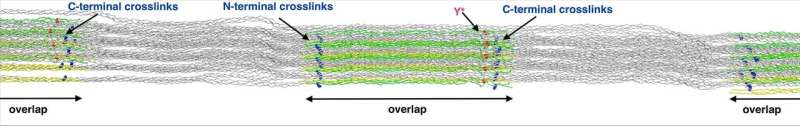
Atomistic model of collagen I fibril with crosslinks (blue) that connect helices. Newly discovered DOPAs are shown in red. Credit: HITS/MBM
It has been known for many decades that synthetic polymers subjected to mechanical stress generate mechanoradicals by rupture of chemical bonds. But could those harmful and highly reactive radicals also form in our tissues when stretched?
Scientists from the Molecular Biomechanics Group at HITS tackled this question by taking a closer look at collagen, the protein which holds us together—literally—and provides structural and mechanical stability to all our connective tissues like bones, tendons, ligaments, and skin. "In this role it is under perpetual mechanical load and as such the perfect candidate," says Frauke Gräter, who led the research at HITS. Together with colleagues from Homburg, Frankfurt and Seattle, her team showed in a series of especially devised experiments that excessive mechanical stress on collagen produces radicals. Knowing that radicals are known to cause damage and oxidative stress in the body, this finding was critical for the researchers.
"We managed to mount and pull a rat tail fascicle directly in the Electron-paramagnetic resonance cavity to monitor radical formation due to force in real time," explains Christopher Zapp, Ph.D. student in Graeter's team, the experimental set-up. Additional Molecular Dynamics simulations of the collagen fibril, comprising millions of atoms, helped to explain the observations: Chemical bonds break when collagen is stretched. But the resulting harmful radicals are quickly scavenged by nearby aromatic residues, so-called DOPAs. "Not only did we find stable radicals in collagen tissue, we also discovered DOPA residues in collagen, a modification that protects collagen against further damage." The DOPA radicals then finally convert into hydrogen peroxide, an important oxidative molecule in the body. Collagen is therefore not just a mere bearer of force, it can also control its consequences.
"It was a challenging task to make sense of the peculiar radical signal we observed in the stressed biomaterial," adds Reinhard Kappl from the Department of Biophysics at Saarland University and co-author of the study. "It needed the combination of expertise from different labs for a consistent picture."
The study suggests that collagen has evolved as a radical sponge to combat damage. "We show that collagen protects itself from the radicals. Still, stretching this mechanism beyond its limits can eventually lead to oxidation-mediated pathologies, from pain to inflammation," explains Agnieszka Obarska-Kosinska from HITS.
The findings might not only explain why playing football can at times be really painful, they are also a promising starting point for improving tissue repair and transplantation, for example in sports medicine.
More information: Christopher Zapp et al. Mechanoradicals in tensed tendon collagen as a source of oxidative stress, Nature Communications (2020). DOI: 10.1038/s41467-020-15567-4
Journal information: Nature Communications
Provided by Heidelberg Institute for Theoretical Studies