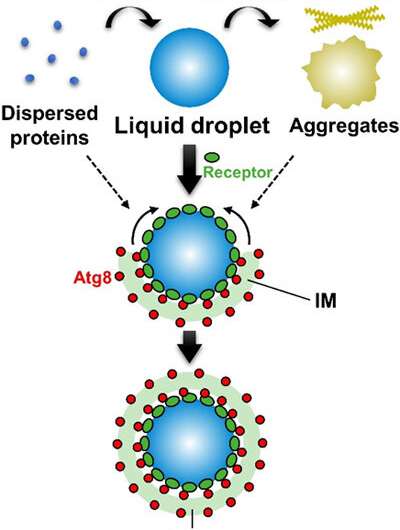
Figure. Selective autophagy efficiently degrades liquid droplets. Some dispersed proteins undergo liquid-liquid phase separation (LLPS) to form liquid droplets. Liquid droplets possess high fluidity and are more similar to a liquid state than a solid state. It is known that liquid droplets lose their liquidity by mutation or stress and are transformed into aggregates and amyloids. Selective autophagy preferentially targets liquid droplets during which receptors accumulate on the surface of droplets and interacts with Atg8 on the isolation membrane (IM), thereby enabling the expansion of the IM along the surface of the droplets. On the other hand, selective autophagy is not good at degrading solid-like aggregates or dispersed proteins. Credit: Institute of Microbial Chemistry
Under JST's Strategic Basic Research Programs, Noda Nobuo and Yamasaki Akinori at the Institute of Microbial Chemistry, in collaboration with other researchers, have discovered that autophagy is effective for selectively degrading protein in a liquid droplet state that is formed through liquid-liquid phase separation, but does poorly with the degradation of protein in aggregation or solid state.
Autophagy is one of the mechanisms through which cellular protein is degraded. There exists a selective type of autophagy called selective autophagy that targets and degrades specific proteins and organelles. It has been assumed that selective autophagy prevents the onset of diseases, but the state of proteins in which they could be efficiently degraded had been unclear.
This research group used the selective autophagy of the Ape1 protein in yeast as the model system and succeeded in reconstituting the Ape1 isolation process with a lipid membrane in a test tube. It revealed that Ape1 protein is effectively isolated by the lipid membrane through the functioning of the Atg8 and receptor proteins when Ape1 forms droplets, but is not isolated by the lipid membrane when Ape1 forms aggregates.
The discovery that selective autophagy is effective in degrading protein droplets but does poorly in degrading protein aggregates indicates that the activation of autophagy alone is insufficient in the prevention of neurodegenerative diseases and other diseases that are believed to be caused by the accumulation of abnormal proteins, and that it is important to develop drugs that change aggregations into droplets. This will hopefully lead to the discovery of drugs that target liquid-liquid phase separation, which is the mechanism for creating droplets.
It was also proven for the first time that the isolation step for the targeted protein in selective autophagy can be conducted with just the Atg8 and receptor proteins. Autophagy also selectively degrades mitochondria and other organelles as well as pathogenic bacteria. Atg8 and receptor proteins could be responsible for selective isolation in a similar mechanism in these cases as well. The application of the test-tube reconstitution system developed here would promote research on the selective autophagy mechanisms for a wide variety of targets.
More information: Akinori Yamasaki et al. Liquidity Is a Critical Determinant for Selective Autophagy of Protein Condensates, Molecular Cell (2020). DOI: 10.1016/j.molcel.2019.12.026
Journal information: Molecular Cell
Provided by Japan Science and Technology Agency