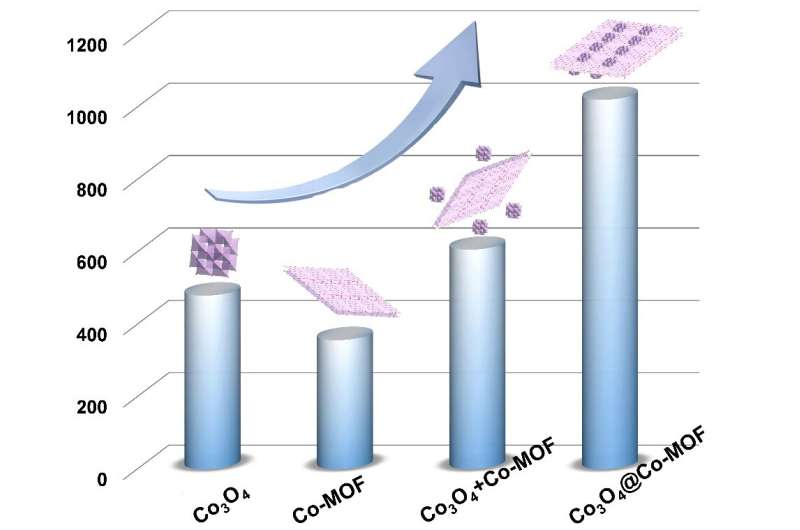
A bar graph for capacitance performance comparison of electrode materials. Credit: ©Science China Press
Metal-organic frameworks (MOFs) are formed via self-assembly of metal ions and organic linkers. Due to their superior properties, such as their large surface area, high porosity and structure tunability, MOFs have recently emerged as one type of important porous materials and have attracted intense interest in many fields, such as gas storage and separation, catalysis, and energy storage. Nevertheless, MOFs still have a few weak points, which impede the use of their full potential to a great extent. For example, most of MOFs manifest inferior properties for electrical conduction and have limited chemical stability (in water, especially alkaline conditions), preventing them from exhibiting their best performance in the field of electrochemistry. Fortunately, hybriding MOFs with a variety of functional materials to generate MOF composites can integrate the merits and mitigate the shortcomings of both parent materials.
Metal oxide nanomaterials with controllable shape, size, crystallinity and functionality are widely applied in many fields. Because of their high theoretical specific capacitance, low cost, and great reversibility, they are considered ideal pseudocapacitive electrode materials, but they have high surface energies and are prone to aggregation, leading to loss of the pseudocapacitive performance. In addition, metal oxides usually display only small surface areas, which has largely restricted the use of metal oxides as electrode materials for electrochemical energy storage. Consequently, finding a cost-effective method to increase the specific surface areas of metal oxides is crucial for achieving high pseudocapacitive activity.
In a new study published in the Beijing-based National Science Review, scientists at Yangzhou University in Yangzhou, China, present a highly alkaline-stable metal oxide@MOF composite, Co3O4 nanocube@Co-MOF (Co3O4@Co-MOF). Co-authors Shasha Zheng, Qing Li, Huaiguo Xue, Huan Pang, and Qiang Xu made a profound statement on the design and synthesis of the Co3O4@Co-MOF, the electrochemical test, and the good prospects of the Co3O4@Co-MOF applied to the electrode of the electrochemical capacitor energy storage device.
The Co3O4@Co-MOF were successfully synthesized via a one-pot hydrothermal reaction under a highly alkaline condition. Without hybriding with Co3O4, Co-MOF can provide an appropriate space for the electrochemical reaction and intercalation/de-intercalation of K+ during the energy storage process, but the alkaline stability of pristine Co-MOF is poor, resulting in capacitance as low as 356 F g-1. The presence of Co3O4 on the surface of Co-MOF effectively improves the alkaline stability, increases redox active sites, leading to dramatic enhancement of capacitance to 1020 F g-1 at 0.5 A g-1. Such a highly alkaline-stable Co3O4@Co-MOF composite shows significant advantages for application as an electrochemical capacitor energy storage device electrode in terms of enhanced durability and capacitance. The Co3O4@Co-MOF composite shows a high cycling stability after 5000 cycles with only 3.3% decay at 5 A g-1. More remarkably, the as-constructed aqueous/solid-state device showed high specific capacitance, wonderful cycle stability, and high energy density. In addition, the as-fabricated solid-state flexible device showed excellent mechanical flexibility and environmental stability. Considering the merits of the facile synthetic method, simple construction and outstanding properties, the Co3O4@Co-MOF//AC solid-state flexible device opens up bright prospects in portable, flexible and lightweight electronic applications.
More information: Shasha Zheng et al, A highly alkaline-stable metal oxide@metal-organic framework composite for high-performance electrochemical energy storage, National Science Review (2019). DOI: 10.1093/nsr/nwz137
Provided by Science China Press