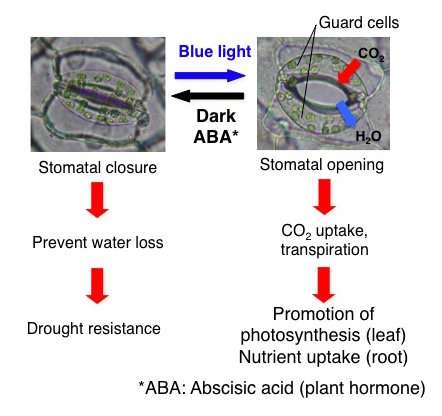
Stomata open in response to blue light, and close in the presence of abscisic acid (ABA), which is biosynthesized when the plant is under dark conditions and/or drought stress. Stomata are essential for the uptake of carbon dioxide (CO2) in order for the plant to carry out photosynthesis. Credit: ITbM, Nagoya University
A team of scientists at Nagoya University has discovered new compounds that can control stomatal movements in plants. Some of the compounds have been shown to prevent leaves from drying up suppressed withering when sprayed on rose and oat leaves. Further investigation could lead to the development of new compounds to extend the freshness of cut flowers and flower bouquets, reduce transportation costs for plants, and to confer drought resistance to crops. The study is published in Plant & Cell Physiology, and explores a new chemical approach that does not rely on classical genetic methods to regulate the stomatal openings in plants.
Stomata are small pores present in the surface of plants, including leaves, flower petals and other organs, and are responsible for gas exchange with the atmosphere. These pores facilitate gas exchange to provide the carbon dioxide necessary for photosynthesis and release water by transpiration, which enhances uptake of nutrients from the roots. Therefore, regulation of stomatal openings is essential for plant growth as well as survival in response to environmental conditions.
Stomata consist of a pair of guard cells and open in response to the blue wavelength present in sunlight, explaining why photosynthesis occurs during the day. When plants are under dark conditions and/or drought stress, a plant hormone, abscisic acid (ABA) is biosynthesized and induces stomata closure. Stomata are closed during the night to prevent water loss from the plant.
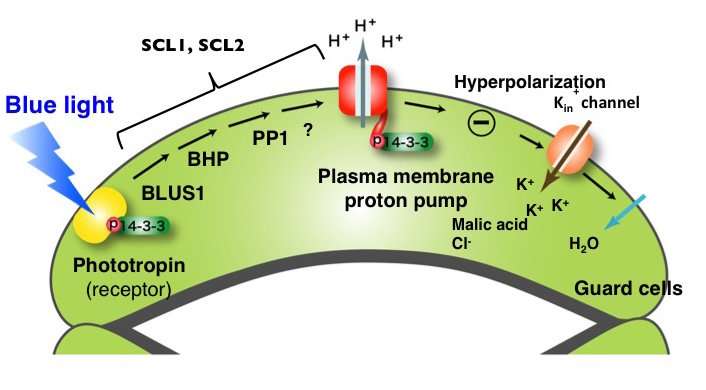
When the guard cells in the stomata detect blue light, the photoreceptor phototropin is activated and induces signaling within the cell. An enzyme called plasma membrane proton pump (PM H+-ATPase) is activated, leading to the uptake of potassium ions. This triggers water uptake and cell swelling that opens the stomata. Although activation of PM H+-ATPase is known to be significant for stomatal opening, the full mechanism of stomatal opening is yet to be clarified.
"As we were interested in revealing the mechanism of stomatal opening, we decided to use a chemical library to explore molecules that affect stomatal opening," says Professor Toshinori Kinoshita, a plant biologist at ITbM and the leader of this study. "We started this research in 2014, and were keen to use a chemistry-based approach instead of classical genetic techniques in order overcome drawbacks such as lethality and redundancy of the genes. We also thought that using compounds to regulate stomatal movements would be useful for developing agrochemicals."
ITbM, which officially started in 2013, has a chemical library managed by Dr. Ayato Sato consisting of both commercial compounds and compounds synthesized by ITbM's chemists. Kinoshita considered this to be a good opportunity to start a new approach.
1) Phototropin receptors in the guard cells of stomata receives blue light; 2) Signal transmission by BLUS1, BHP and PP1 (proposed signaling components); 3) Activation of the plasma membrane proton pump (PM H+-ATPase); 4) Potassium (K+) ion uptake; 5) Accumulation of K+ ions increases the osmotic pressure in the guard cells; 6) Water uptake and swelling of cells; 7) Stomatal opening. This study has shown that compounds SCL1 and SCL2 act upon the signaling factors between phototropin and PM H+-ATPase. Credit: ITbM, Nagoya University
Using the herb Benghal dayflower as a model plant, Dr. Shigeo Toh, Mr. Shinpei Inoue, and Dr. Yosuke Toda established experimental conditions to screen over 20,000 compounds. They managed to find hit compounds after a year of random screening. This includes nine compounds that suppress light-induced stomatal opening by more than 50 percent, and two compounds that induce stomatal opening even in the dark.
"The key to this research was to screen and find hit compounds as efficiently as possible from a vast pool of compounds," describes Kinoshita.
Further analysis of stomatal closing compounds (SCLs), SCL1 and SCL2 revealed that that they inhibit the signaling components between the phototropin receptor and the PM H+-ATPase enzyme, thus inhibiting light-induced activation of PM H+-ATPase and leading to suppression of stomatal opening.
The researchers also sprayed SCL1 on rose and oat leaves and found that withering of the leaves was successfully suppressed over a certain time period.
"This was the best moment in our research, to find that the molecules that we had discovered had an effect on suppressing the withering of leaves," says Kinoshita. "The fact these compounds induce stomatal closure by a different mechanism to the plant hormone, ABA is important."
Along with its stomatal closure effect in response to drought stress, ABA is also known to be an inhibitor for seed germination and plant growth. Although ABA and its derivatives are currently being developed as reagents to induce drought tolerance to plants, their side effects have been an issue. SCL1 currently shows to have an effect only on stomatal closure, so the team envisages that this is a good starting point for the development of new reagents for drought tolerance. They consider that these types of compounds would be useful for keeping cut flowers fresh and also reduce transportation costs, simply by spraying the compounds on the plants.
"We hope to be able to find partners to work on further elucidation of the stomatal opening mechanism and developing compounds together that can be applied for drought tolerance to various plants, including major crops and flowers," says Kinoshita. "We also believe that by making leaves last for longer, this may lead to increasing the variation of leaves that can be used in the Japanese traditional flower arrangement, Ikebana, which currently has a tendency to avoid using simple leaves that can easily wither."
More information: Shigeo Toh et al, Identification and Characterization of Compounds that Affect Stomatal Movements, Plant and Cell Physiology (2018). DOI: 10.1093/pcp/pcy061
Journal information: Plant and Cell Physiology