Plants use receptor kinases as molecular antennas to monitor their environment but, strangely, one of them is actively cleaved. A new study discovered that one of the resulting pieces interacts with a receptor for bacterial signals.
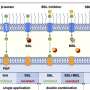
Like antennas, a variety of so-called receptor kinases stick out of the plant cell membrane into the surrounding medium. They are thus well placed to perceive signals in the immediate vicinity. Some of these receptor kinases are crucial for a proper cross-talk between plants and beneficial bacteria or fungi. Plants belonging to the legume family – which includes peas, beans and soybeans – form special structures called root nodules to accommodate their nitrogen-fixing bacterial symbionts. "The plant identifies symbiotic bacteria with the help of receptor kinases" says LMU geneticist Martin Parniske.
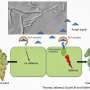
The bacteria produce chemical signals - so called nodulation factors - that are recognized by plant nodulation factor receptors. This initiates a signalling process within the root cell, which leads to organized cell proliferation and the formation of new organs, the root nodules. "We knew for many years that the symbiosis receptor kinase SYMRK is essential in this signalling process although it does not seem to directly bind the bacterial nodulation factors. And in the course of investigating how SYMRK actually works, we made an astonishing discovery," says Parniske,whose research group studies the molecular processes underlying plant root symbioses.
Cleaved SYMRK interacts with the nodulation factor receptor
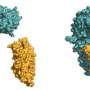
The extracellular domain of SYMRK is made up of two structural modules. "In between these two modules, the antenna gets cleaved. This is what we found when we had a closer look at the extracellular portion of SYMRK. It ends up as two pieces," Parniske explains. The cleavage drastically alters the properties of the receptor. Indeed, the membrane-bound fragment produced by the cleavage forms a complex with one of the nodulation factor receptors.
"This was a completely unexpected finding, because no such cleavage of a receptorkinase had ever been observed in plants before," says Parniske.The cleavage also destabilizes the part of SYMRK that remains anchored in the membrane and interacts with the nodulation factor receptor. The researchers speculate that this might lead to the inactivation of the receptor complex as a whole. This mechanism may serve to ensure that the symbiotic infection remains localized in extent and restricted to a narrow time window.
"Receptor kinases like SYMRK are widespread in the plant kingdom, and any given species can have up to several hundred different types. The functions of the vast majority of these receptors remain unknown," says Parniske. "The question now is whether this form of receptor cleavage, which was previously known only from animal receptors, is a more widespread feature of receptor kinases in plants" he concludes.
More information: "Cleavage of the SYMBIOSIS RECEPTOR-LIKE KINASE Ectodomain Promotes Complex Formation with Nod Factor Receptor 5." Meritxell Antolín-Llovera, Martina K. Ried, Martin Parniske. Current Biology - 17 February 2014 (Vol. 24, Issue 4, pp. 422-427)
Journal information: Current Biology
Provided by Ludwig Maximilian University of Munich