The debut of cyborgs who are part human and part machine may be a long way off, but researchers say they now may be getting closer. In a study published in ACS' journal Nano Letters, they report development of a coating that makes nanoelectronics much more stable in conditions mimicking those in the human body. The advance could also aid in the development of very small implanted medical devices for monitoring health and disease.
Charles Lieber and colleagues note that nanoelectronic devices with nanowire components have unique abilities to probe and interface with living cells. They are much smaller than most implanted medical devices used today. For example, a pacemaker that regulates the heart is the size of a U.S. 50-cent coin, but nanoelectronics are so small that several hundred such devices would fit in the period at the end of this sentence. Laboratory versions made of silicon nanowires can detect disease biomarkers and even single virus cells, or record heart cells as they beat. Lieber's team also has integrated nanoelectronics into living tissues in three dimensions—creating a "cyborg tissue." One obstacle to the practical, long-term use of these devices is that they typically fall apart within weeks or days when implanted. In the current study, the researchers set out to make them much more stable.
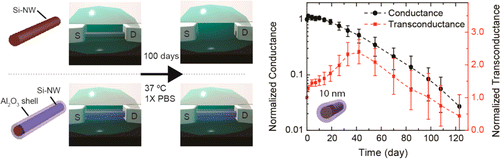
They found that coating silicon nanowires with a metal oxide shell allowed nanowire devices to last for several months. This was in conditions that mimicked the temperature and composition of the inside of the human body. In preliminary studies, one shell material appears to extend the lifespan of nanoelectronics to about two years.
More information: "Long Term Stability of Nanowire Nanoelectronics in Physiological Environments" Nano Lett., Article ASAP. DOI: 10.1021/nl500070h
Abstract
Nanowire nanoelectronic devices have been exploited as highly sensitive subcellular resolution detectors for recording extracellular and intracellular signals from cells, as well as from natural and engineered/cyborg tissues, and in this capacity open many opportunities for fundamental biological research and biomedical applications. Here we demonstrate the capability to take full advantage of the attractive capabilities of nanowire nanoelectronic devices for long term physiological studies by passivating the nanowire elements with ultrathin metal oxide shells. Studies of Si and Si/aluminum oxide (Al2O3) core/shell nanowires in physiological solutions at 37 °C demonstrate long-term stability extending for at least 100 days in samples coated with 10 nm thick Al2O3 shells. In addition, investigations of nanowires configured as field-effect transistors (FETs) demonstrate that the Si/Al2O3 core/shell nanowire FETs exhibit good device performance for at least 4 months in physiological model solutions at 37 °C. The generality of this approach was also tested with in studies of Ge/Si and InAs nanowires, where Ge/Si/Al2O3 and InAs/Al2O3 core/shell materials exhibited stability for at least 100 days in physiological model solutions at 37 °C. In addition, investigations of hafnium oxide-Al2O3 nanolaminated shells indicate the potential to extend nanowire stability well beyond 1 year time scale in vivo. These studies demonstrate that straightforward core/shell nanowire nanoelectronic devices can exhibit the long term stability needed for a range of chronic in vivo studies in animals as well as powerful biomedical implants that could improve monitoring and treatment of disease.
Journal information: Nano Letters
Provided by American Chemical Society