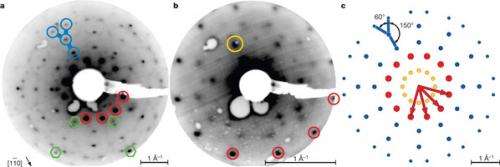
Electron diffraction from dodecagonal oxide thin film. Credit: Nature 502, 215–218 (10 October 2013) doi:10.1038/nature12514
(Phys.org) —A team of researchers working at Germany's Martin-Luther-Universität has discovered a new form of a 12-sidded quasicrystal. In their paper published in the journal Nature, the team describes how they accidently created the previously unknown crystalline structured material while investigating interfacing properties between various substances.
Quasicrystals are substances that look a lot like crystals but have one major exception—the pattern of their structure is non-repeating. They were first discovered in 1982 by Daniel Shechtman—he won the Nobel Prize in chemistry for it in 2011. Since that time they have been created in the lab in various ways and have even been found in nature—as part of a meteorite that fell in Russia (which because it was found to have been created by a non-heat related astrophysical process, showed that applying heat wasn't necessary to create them). In this latest effort the researchers created one using perovskite oxides, potentially extending the number of materials that can be created by such substances.
The team in Germany was investigating the ways perovskite behaved when used as a layer on top of a metal base. After exposure to extremely high temperatures, they noted that the material began to shape into a pattern, which they naturally assumed was a crystal. Upon closer inspection, they found that the 12-sided pattern didn't repeat itself—the mark of a quasicrystal. The team notes that perovskite oxides are not normally noted for forming into quasicrystals, and in fact, no one really thought it was possible.
The discovery extends the types of quasicrystals that are known to exist, though not all of them have 12 sides of course. Their unusual structures make possible the creation of materials with unusual properties which scientists are just now beginning to find. Finding ways to create them using materials not normally associated with such odd structures may pave the way to a much broader array of end products—now that scientists know that it is possible, the door has been opened to creating all sorts of new materials from perovskite oxide based quasicrystals (now called barium titanate), such as thermal insulators or coatings for electronic components.
More information: Quasicrystalline structure formation in a classical crystalline thin-film system, Nature 502, 215–218 (10 October 2013) DOI: 10.1038/nature12514
Abstract
The discovery of quasicrystals—crystalline structures that show order while lacking periodicity—forced a paradigm shift in crystallography. Initially limited to intermetallic systems, the observation of quasicrystalline structures has recently expanded to include 'soft' quasicrystals in the fields of colloidal and supermolecular chemistry. Here we report an aperiodic oxide that grows as a two-dimensional quasicrystal on a periodic single-element substrate. On a Pt(111) substrate with 3-fold symmetry, the perovskite barium titanate BaTiO3 forms a high-temperature interface-driven structure with 12-fold symmetry. The building blocks of this dodecagonal structure assemble with the theoretically predicted Stampfli–Gähler tiling having a fundamental length-scale of 0.69?nm. This example of interface-driven formation of ultrathin quasicrystals from a typical periodic perovskite oxide potentially extends the quasicrystal concept to a broader range of materials. In addition, it demonstrates that frustration at the interface between two periodic materials can drive a thin film into an aperiodic quasicrystalline phase, as proposed previously. Such structures might also find use as ultrathin buffer layers for the accommodation of large lattice mismatches in conventional epitaxy.
Journal information: Nature
© 2013 Phys.org