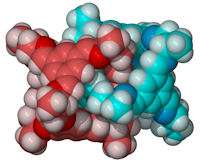
The molecular `knots' have dimensions of around two nanometers
Scientists at the University of Liverpool have constructed molecular 'knots' with dimensions of around two nanometers -- around 30,000 times smaller than the diameter of a human hair.
Most molecules are held together by chemical bonds between atoms - 'nano-knots' are instead mechanically bonded by interpenetrating loops. Liverpool scientists have managed to create nanoscale knots in the laboratory by mixing together two simple starting materials - one a rigid aromatic compound and the other a more flexible amine linker.
This is an unusual example of 'self-assembly', a process which underpins biology and allows complex structures to assemble from more simple building blocks. Each knot is 'tied' three times: that is, at least three chemical bonds must be broken to untie the knot. A single knot is a complex assembly of 20 smaller molecules.
Professor Andrew Cooper, Director of the University's Centre for Materials Discovery, said: "I was amazed when we discovered these molecules; we actually set out to make something simpler. A complex structure arises out of quite basic building blocks.
"It is like shaking Scrabble tiles in a bag and pulling out a fully formed sentence. These are the surprises which make scientific research so fascinating."
The experimental work was led by Dr Tom Hasell, a Postdoctoral Researcher, who recognized that the data in an experiment to create organic nanocages was anomalous. In particular, the mass of the molecules was twice as high as expected, a result of the complex mechanical interlocking of two molecular sub-units. The team is now focusing on the practical application of these molecules and similar structures - for example, to build molecular 'machines' which can trap harmful gases and pollutants such as carbon dioxide.
The research, which was published in the journal Nature Chemistry, forms part of a broader five-year programme focusing on the synthesis of new materials for applications such as energy storage and conversion.
Provided by University of Liverpool